Corporate Presentation
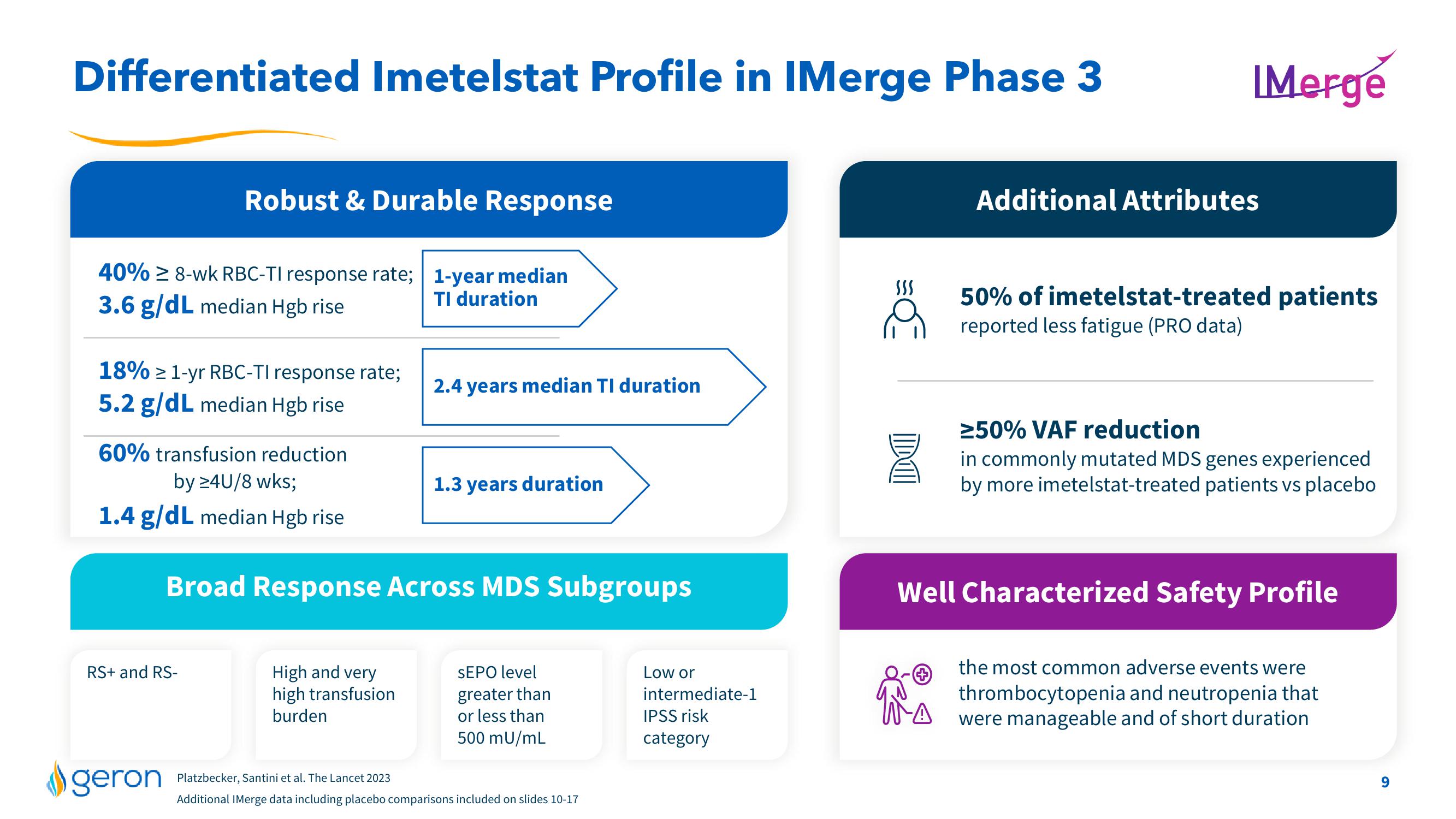
Differentiated Imetelstat Profile in IMerge Phase 3
40% ≥ 8-wk RBC-TI response rate; 1-year median
3.6 g/dL median Hgb rise
TI duration
Robust & Durable Response
18% ≥ 1-yr RBC-TI response rate;
5.2 g/dL median Hgb rise
60% transfusion reduction
by ≥4U/8 wks;
1.4 g/dL median Hgb rise
RS+ and RS-
geron
2.4 years median TI duration
Broad Response Across MDS Subgroups
High and very
high transfusion
burden
1.3 years duration
SEPO level
greater than
or less than
500 mU/mL
Platzbecker, Santini et al. The Lancet 2023
Additional IMerge data including placebo comparisons included on slides 10-17
Low or
intermediate-1
IPSS risk
category
SSS
IDA
IMerge
Additional Attributes
50% of imetelstat-treated patients
reported less fatigue (PRO data)
≥50% VAF reduction
in commonly mutated MDS genes experienced
by more imetelstat-treated patients vs placebo
Well Characterized Safety Profile
the most common adverse events were
thrombocytopenia and neutropenia that
were manageable and of short duration
9View entire presentation