Equillium Results Presentation Deck
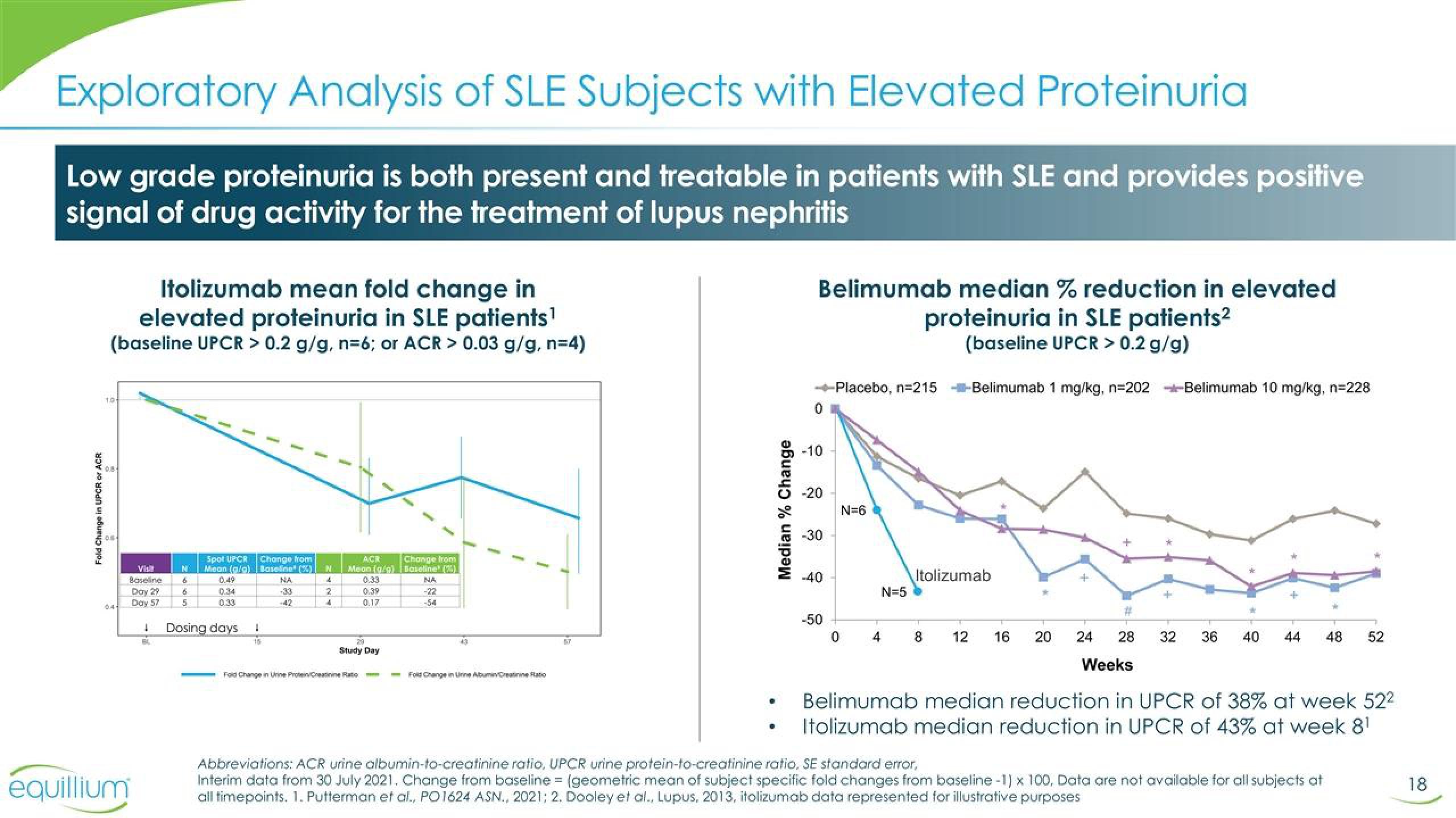
Exploratory Analysis of SLE Subjects with Elevated Proteinuria
Low grade proteinuria is both present and treatable in patients with SLE and provides positive
signal of drug activity for the treatment of lupus nephritis
Fold Change in UPCR or ACR
Itolizumab mean fold change in
elevated proteinuria in SLE patients¹
(baseline UPCR > 0.2 g/g, n=6; or ACR> 0.03 g/g, n=4)
1.0
Os
equillium
Baseline
Day 29
Day 57
N
6
6
5
Spot UPCR Change from
Mean (n/a)
0.49
Baseline (3)
NA
į Dosing days I
+42
N
4
2
4
ACR
Change from
Meon (g/n) Baseline (25)
0.39
0.17
Study Day
Fold Change in Unne Protein Create Rato
-54
43
Fold Change in Urine AlbumCreate a
4
Median % Change
Belimumab median % reduction in elevated
proteinuria in SLE patients²
(baseline UPCR > 0.2 g/g)
-Belimumab 1 mg/kg, n=202 Belimumab 10 mg/kg, n=228
0
-10
-20
-30
-40
-50
Placebo, n=215
0
N=6
4
N=5
Itolizumab
8 12
#
16 20 24 28 32 36 40 44 48 52
Weeks
Belimumab median reduction in UPCR of 38% at week 52²
Itolizumab median reduction in UPCR of 43% at week 8¹
Abbreviations: ACR urine albumin-to-creatinine ratio, UPCR urine protein-to-creatinine ratio, SE standard error,
Interim data from 30 July 2021. Change from baseline = (geometric mean of subject specific fold changes from baseline -1) x 100, Data are not available for all subjects at
all timepoints. 1. Putterman et al., PO1624 ASN., 2021; 2. Dooley et al., Lupus, 2013, itolizumab data represented for illustrative purposes
18View entire presentation