Dare Bioscience Investor Presentation Deck
Ovaprene® Investigational Hormone-Free, Monthly Contraceptive
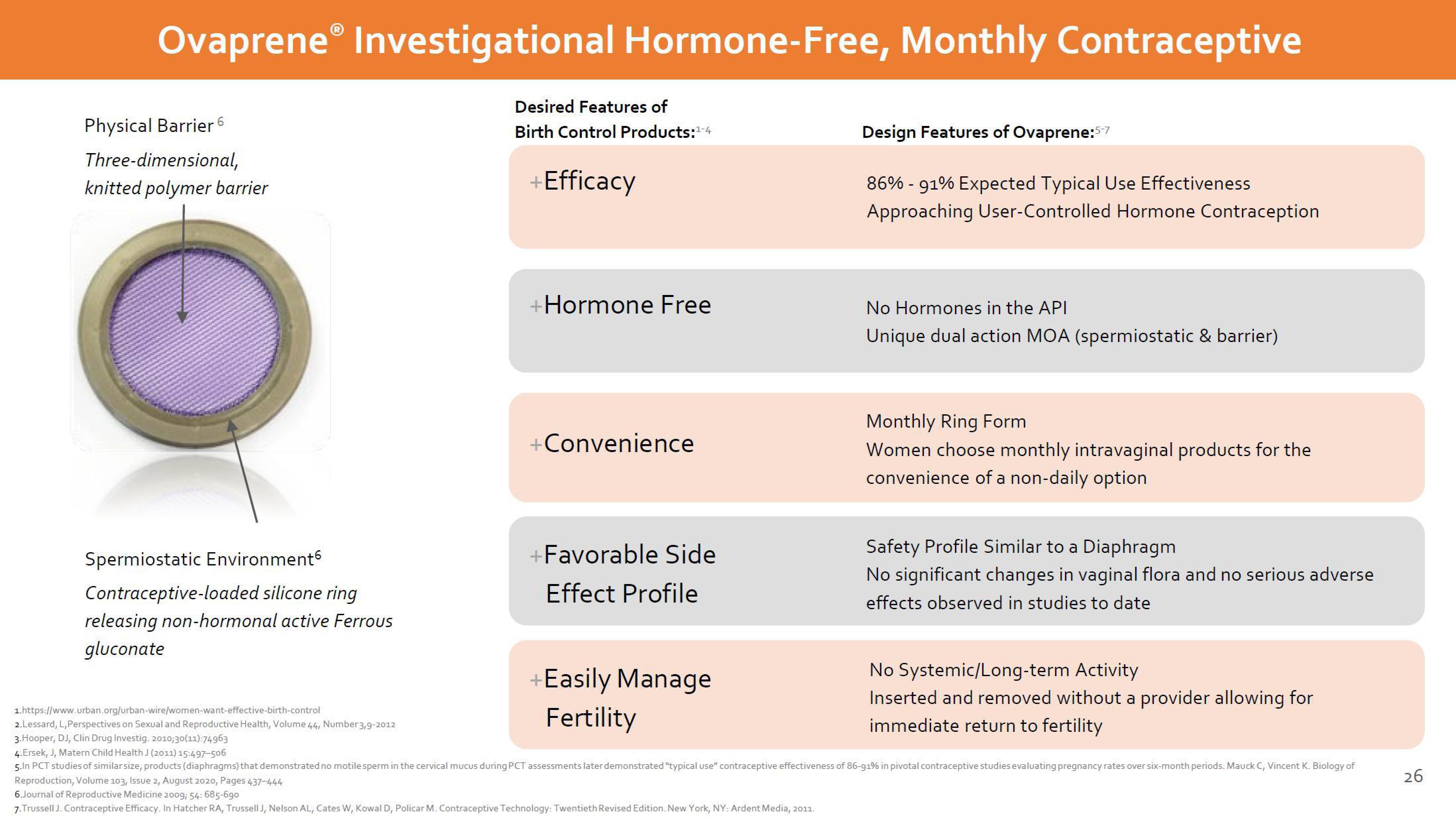
Physical Barrier 6
Three-dimensional,
knitted polymer barrier
Spermiostatic Environment
Contraceptive-loaded silicone ring
releasing non-hormonal active Ferrous
gluconate
Desired Features of
Birth Control Products:¹-4
+Efficacy
+Hormone Free
+Convenience
+Favorable Side
Effect Profile
+
Easily Manage
Fertility
Design Features of Ovaprene: 5-7
86% - 91% Expected Typical Use Effectiveness
Approaching User-Controlled Hormone Contraception
No Hormones in the API
Unique dual action MOA (spermiostatic & barrier)
Monthly Ring Form
Women choose monthly intravaginal products for the
convenience of a non-daily option
Safety Profile Similar to a Diaphragm
No significant changes in vaginal flora and no serious adverse
effects observed in studies to date
No Systemic/Long-term Activity
Inserted and removed without a provider allowing for
immediate return to fertility
1.https://www.urban.org/urban-wire/women-want-effective-birth-control
2.Lessard, L, Perspectives on Sexual and Reproductive Health, Volume 44, Number 3,9-2012
3.Hooper, DJ, Clin Drug Investig. 2010;30(11):74963
4.Ersek, J, Matern Child Health J (2011) 15:497-506
5.In PCT studies of similarsize, products (diaphragms) that demonstrated no motile sperm in the cervical mucus during PCT assessments later demonstrated "typical use" contraceptive effectiveness of 86-91% in pivotal contraceptive studies evaluating pregnancy rates over six-month periods. Mauck C, Vincent K. Biology of
Reproduction, Volume 103, Issue 2, August 2020, Pages 437-444
6. Journal of Reproductive Medicine 2009; 54: 685-690
7. Trussell J. Contraceptive Efficacy. In Hatcher RA, Trussell J, Nelson AL, Cates W, Kowal D, Policar M. Contraceptive Technology: Twentieth Revised Edition. New York, NY: Ardent Media, 2011.
26View entire presentation