BenevolentAI Investor Presentation Deck
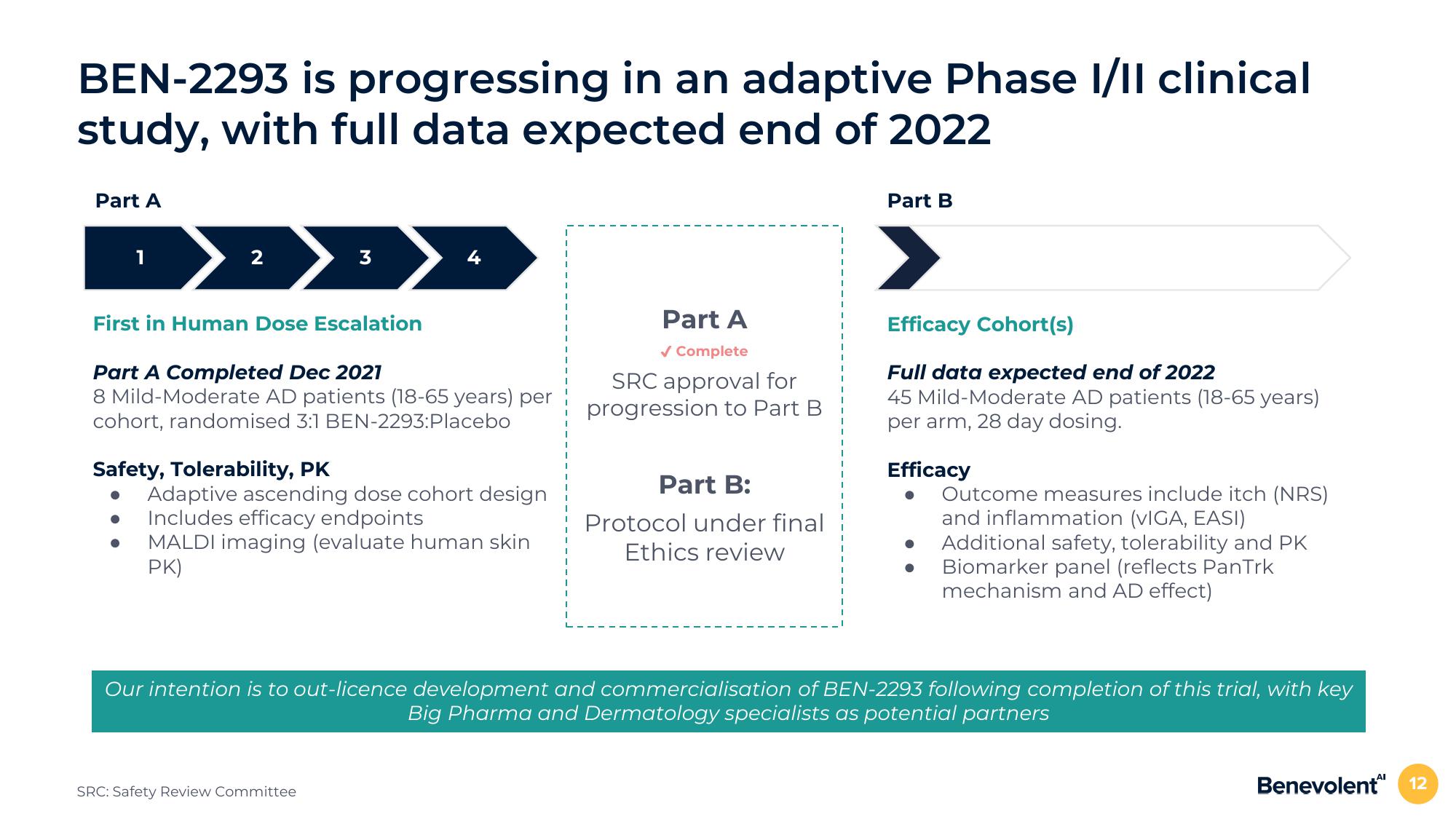
BEN-2293 is progressing in an adaptive Phase I/II clinical
study, with full data expected end of 2022
Part A
1
2
First in Human Dose Escalation
3
Part A Completed Dec 2021
8 Mild-Moderate AD patients (18-65 years) per
cohort, randomised 3:1 BEN-2293:Placebo
Safety, Tolerability, PK
Adaptive ascending dose cohort design
Includes efficacy endpoints
MALDI imaging (evaluate human skin
PK)
SRC: Safety Review Committee
Part A
✓ Complete
SRC approval for
progression to Part B
Part B:
Protocol under final
Ethics review
Part B
Efficacy Cohort(s)
Full data expected end of 2022
45 Mild-Moderate AD patients (18-65 years)
per arm, 28 day dosing.
Efficacy
Outcome measures include itch (NRS)
and inflammation (VIGA, EASI)
Additional safety, tolerability and PK
Biomarker panel (reflects PanTrk
mechanism and AD effect)
Our intention is to out-licence development and commercialisation of BEN-2293 following completion of this trial, with key
Big Pharma and Dermatology specialists as potential partners
Benevolent 12View entire presentation