BioAtla IPO Presentation Deck
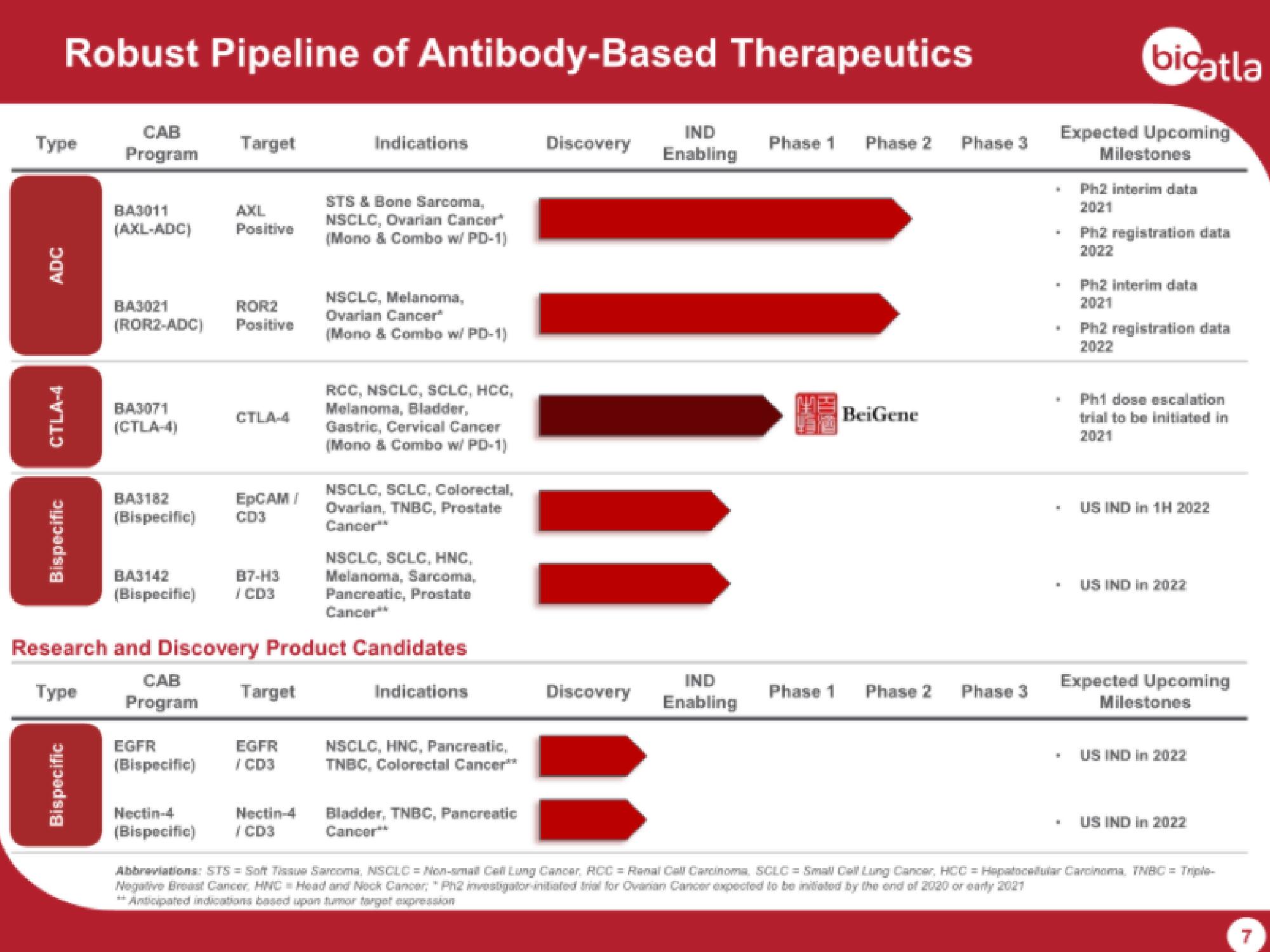
Type
ADC
Robust Pipeline of Antibody-Based Therapeutics
CAB
Program
CTLA-4
Bispecific
Bispecific
BA3011
AXL
(AXL-ADC) Positive
BA3021
ROR2
(ROR2-ADC) Positive
BA3071
(CTLA-4)
Target
BA3182
(Bispecific)
CTLA-4
EGFR
(Bispecific)
EpCAM /
CD3
BA3142
87-H3
(Bispecific) / CD3
Target
EGFR
/CD3
Nectin-4
(Bispecific) /CD3
Nectin-4
Indications
Research and Discovery Product Candidates
Туре
CAB
Program
Indications
STS & Bone Sarcoma,
NSCLC, Ovarian Cancer
(Mono & Combo w/ PD-1)
NSCLC, Melanoma,
Ovarian Cancer
(Mono & Combo w/ PD-1)
RCC, NSCLC, SCLC, HCC,
Melanoma, Bladder,
Gastric, Cervical Cancer
(Mono & Combo w/ PD-1)
NSCLC, SCLC, Colorectal,
Ovarian, TNBC, Prostate
Cancer**
NSCLC, SCLC, HNC,
Melanoma, Sarcoma,
Pancreatic, Prostate
Cancer**
NSCLC, HNC, Pancreatic,
TNBC, Colorectal Cancer**
Bladder, TNBC, Pancreatic
Cancer**
Discovery
Discovery
IND
Enabling
IND
Enabling
Phase 1
Phase 1
Phase 2
BeiGene
Phase 2
Phase 3
Phase 3
H
Expected Upcoming
■
"
H
"
I
bicatla
Milestones
H
Ph2 interim data
2021
Ph2 registration data
2022
H Ph1 dose escalation
trial to be initiated in
2021
Ph2 interim data
2021
Ph2 registration data
2022
US IND in 1H 2022
US IND In 2022
Expected Upcoming
Milestones
US IND in 2022
" US IND In 2022
Abbreviations: STS = Soft Tissua Sarcoma, NSCLC = Non-small Call Lung Cancer, RCC = Renal Call Carcinoma, SCLC = Small Call Lung Cancer, HCC = Hapatocellular Carcinoma, TNBC = Triple-
Negative Breast Cancer, HWC-Head and Neck Cancer: "Ph2 investigator initiated trial for Ovarian Cancer expected to be initiated by the end of 2020 or early 2021
** Anticipated indications based upon tumor target expression
7View entire presentation