OmniAb Investor Presentation Deck
FcRn
●
●
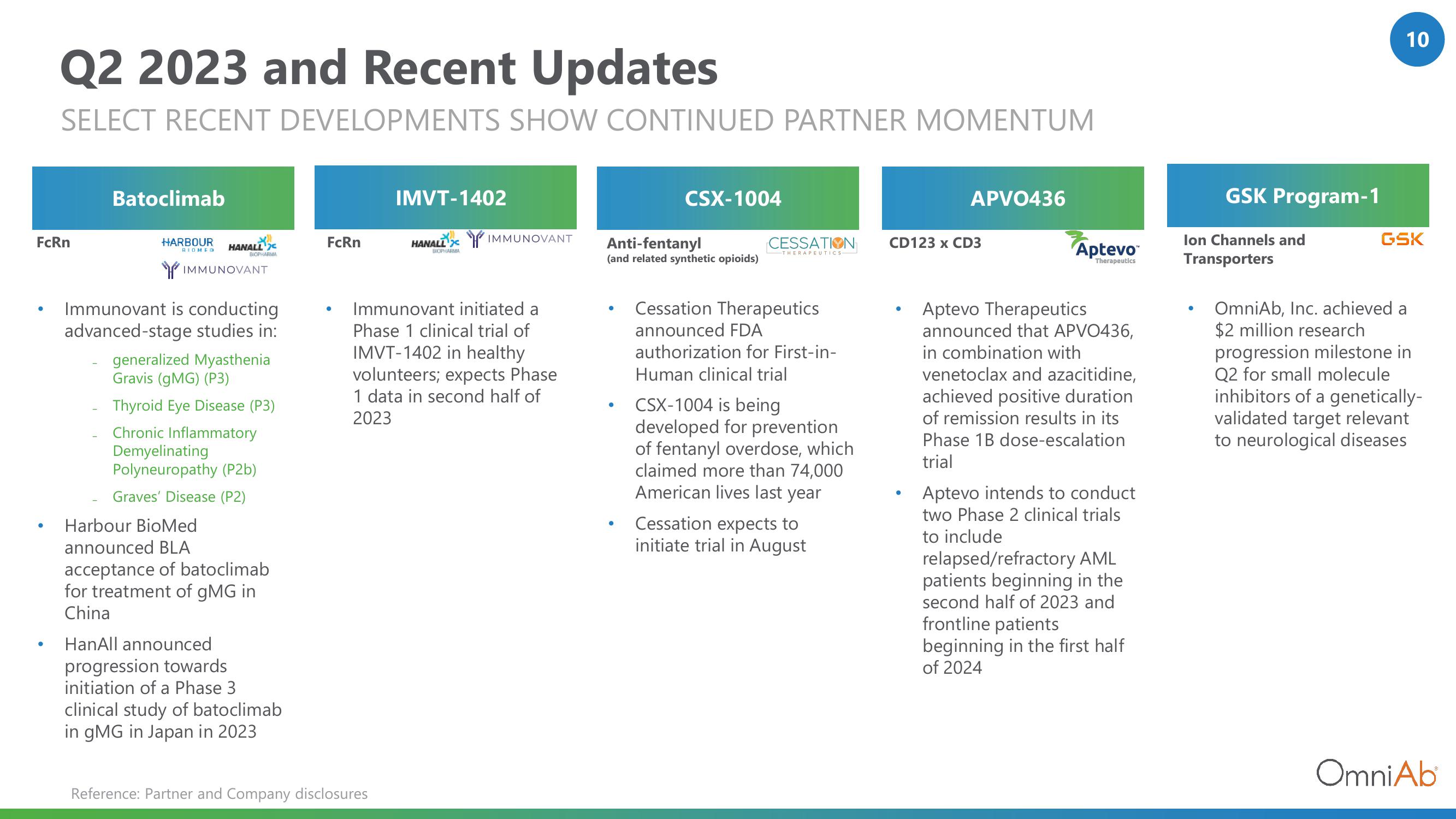
Q2 2023 and Recent Updates
SELECT RECENT DEVELOPMENTS SHOW CONTINUED PARTNER MOMENTUM
●
Batoclimab
HARBOUR
BIOMED
HANALL><
BIOPHARMA
IMMUNOVANT
Immunovant is conducting
advanced-stage studies in:
generalized Myasthenia
Gravis (gMG) (P3)
Thyroid Eye Disease (P3)
Chronic Inflammatory
Demyelinating
Polyneuropathy (P2b)
Graves' Disease (P2)
Harbour BioMed
announced BLA
acceptance of batoclimab
for treatment of gMG in
China
HanAll announced
progression towards
initiation of a Phase 3
clinical study of batoclimab
in gMG in Japan in 2023
FcRn
●
IMVT-1402
Reference: Partner and Company disclosures
HANALLX IMMUNOVANT
BIOPHARMA
Immunovant initiated a
Phase 1 clinical trial of
IMVT-1402 in healthy
volunteers; expects Phase
1 data in second half of
2023
CSX-1004
Anti-fentanyl
(and related synthetic opioids)
●
CESSATION CD123 x CD3
-THERAPEUT
CSI
Cessation Therapeutics
announced FDA
authorization for First-in-
Human clinical trial
CSX-1004 is being
developed for prevention
of fentanyl overdose, which
claimed more than 74,000
American lives last year
Cessation expects to
initiate trial in August
APV0436
Aptevo™
Therapeutics
Aptevo Therapeutics
announced that APVO436,
in combination with
venetoclax and azacitidine,
achieved positive duration
of remission results in its
Phase 1B dose-escalation
trial
Aptevo intends to conduct
two Phase 2 clinical trials
to include
relapsed/refractory AML
patients beginning in the
second half of 2023 and
frontline patients
beginning in the first half
of 2024
GSK Program-1
Ion Channels and
Transporters
10
GSK
OmniAb, Inc. achieved a
$2 million research
progression milestone in
Q2 for small molecule
inhibitors of a genetically-
validated target relevant
to neurological diseases
OmniAbView entire presentation