argenx SE Investor Day Presentation Deck
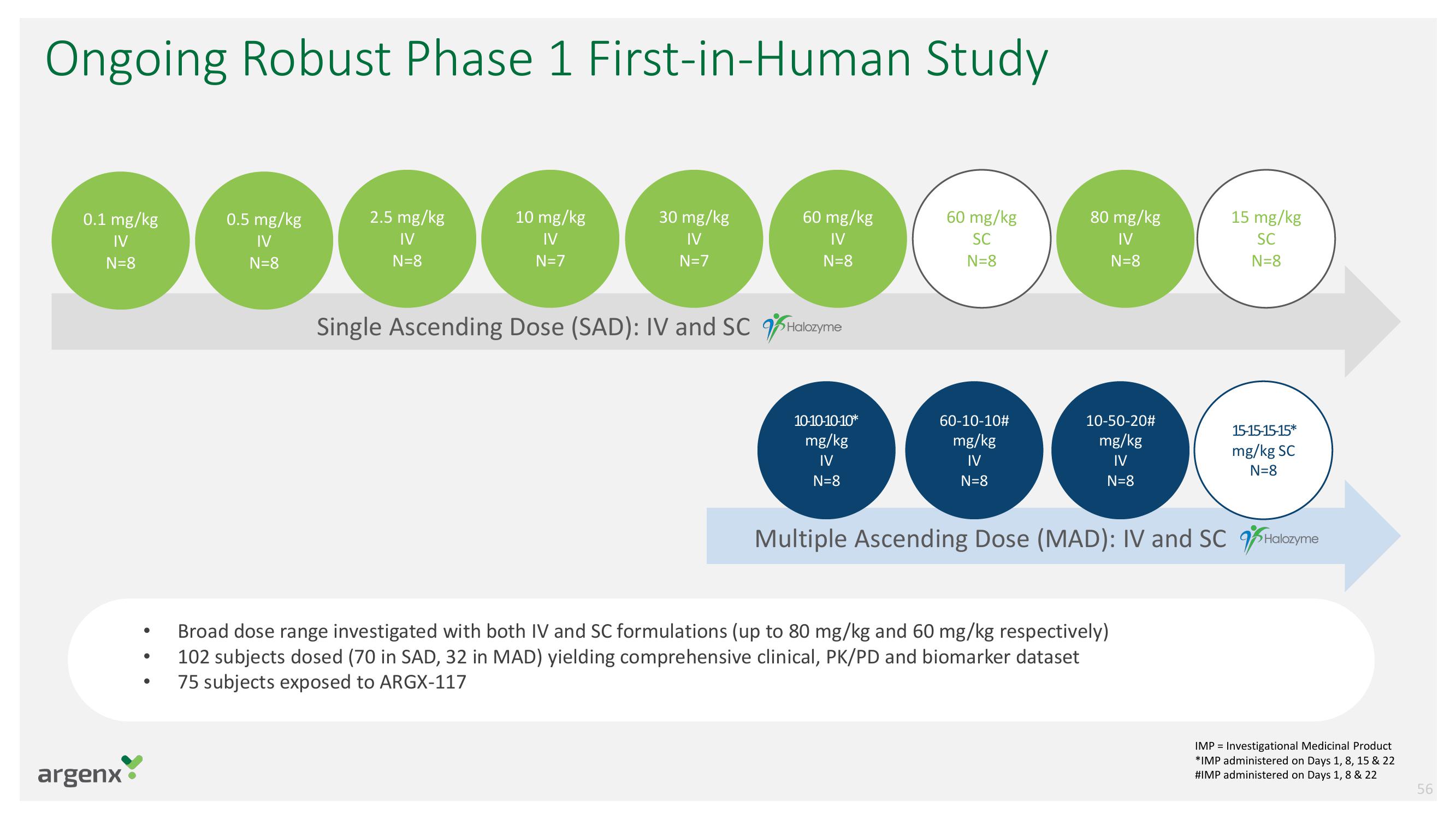
Ongoing Robust Phase 1 First-in-Human Study
0.1 mg/kg
IV
N=8
argenx
●
●
●
0.5 mg/kg
IV
N=8
10 mg/kg
IV
N=7
30 mg/kg
IV
N=7
Single Ascending Dose (SAD): IV and SC Halozyme
2.5 mg/kg
IV
N=8
60 mg/kg
IV
N=8
10-10-10-10*
mg/kg
IV
N=8
60 mg/kg
SC
N=8
60-10-10#
mg/kg
IV
N=8
80 mg/kg
IV
N=8
10-50-20#
mg/kg
IV
N=8
15 mg/kg
SC
N=8
Broad dose range investigated with both IV and SC formulations (up to 80 mg/kg and 60 mg/kg respectively)
102 subjects dosed (70 in SAD, 32 in MAD) yielding comprehensive clinical, PK/PD and biomarker dataset
75 subjects exposed to ARGX-117
15-15-15-15*
mg/kg SC
N=8
Multiple Ascending Dose (MAD): IV and SC Halozyme
IMP= Investigational Medicinal Product
*IMP administered on Days 1, 8, 15 & 22
#IMP administered on Days 1, 8 & 22
56View entire presentation