Kymera Investor Presentation Deck
●
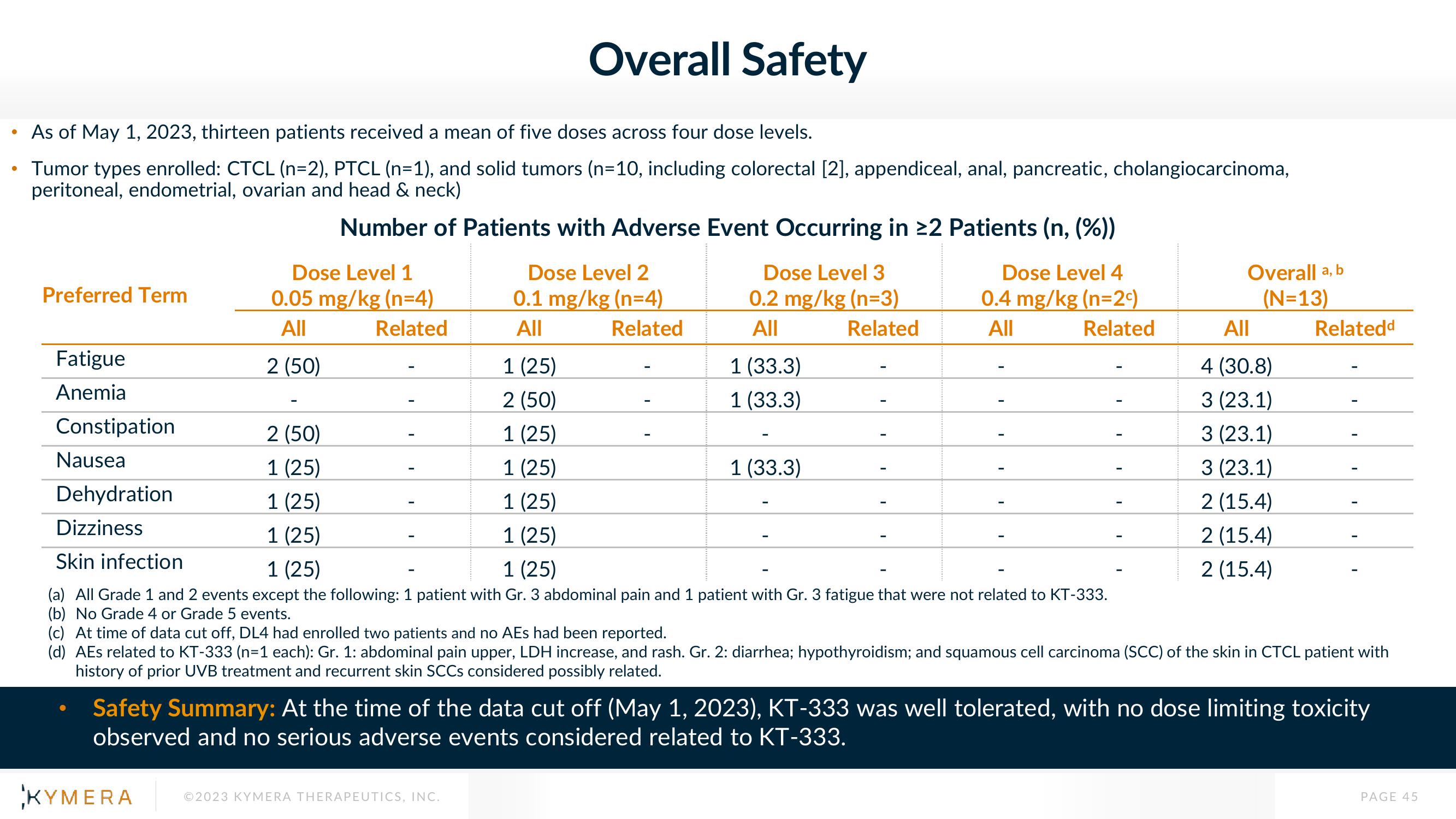
As of May 1, 2023, thirteen patients received a mean of five doses across four dose levels.
• Tumor types enrolled: CTCL (n=2), PTCL (n=1), and solid tumors (n=10, including colorectal [2], appendiceal, anal, pancreatic, cholangiocarcinoma,
peritoneal, endometrial, ovarian and head & neck)
Number of Patients with Adverse Event Occurring in ≥2 Patients (n, (%))
Preferred Term
Fatigue
Anemia
Constipation
Nausea
Dehydration
Dizziness
Overall Safety
Dose Level 1
0.05 mg/kg (n=4)
All
Related
2 (50)
Dose Level 2
0.1 mg/kg (n=4)
All
Related
Dose Level 3
0.2 mg/kg (n=3)
All
Related
KYMERA Ⓒ2023 KYMERA THERAPEUTICS, INC.
1 (25)
2 (50)
2 (50)
1 (25)
1 (25)
1 (25)
1 (25)
1 (25)
1 (25)
1 (25)
1 (25)
Skin infection
1 (25)
(a) All Grade 1 and 2 events except the following: 1 patient with Gr. 3 abdominal pain and 1 patient with Gr. 3 fatigue that were not related to KT-333.
(b) No Grade 4 or Grade 5 events.
1 (33.3)
1 (33.3)
1 (33.3)
I
Dose Level 4
0.4 mg/kg (n=2c)
Related
All
I
I
I
I
Overall a, b
(N=13)
All
4 (30.8)
3 (23.1)
3 (23.1)
3 (23.1)
2 (15.4)
2 (15.4)
2 (15.4)
Relatedd
(c) At time of data cut off, DL4 had enrolled two patients and no AEs had been reported.
(d) AEs related to KT-333 (n=1 each): Gr. 1: abdominal pain upper, LDH increase, and rash. Gr. 2: diarrhea; hypothyroidism; and squamous cell carcinoma (SCC) of the skin in CTCL patient with
history of prior UVB treatment and recurrent skin SCCs considered possibly related.
Safety Summary: At the time of the data cut off (May 1, 2023), KT-333 was well tolerated, with no dose limiting toxicity
observed and no serious adverse events considered related to KT-333.
PAGE 45View entire presentation