Nuvectis Pharma Investor Presentation Deck
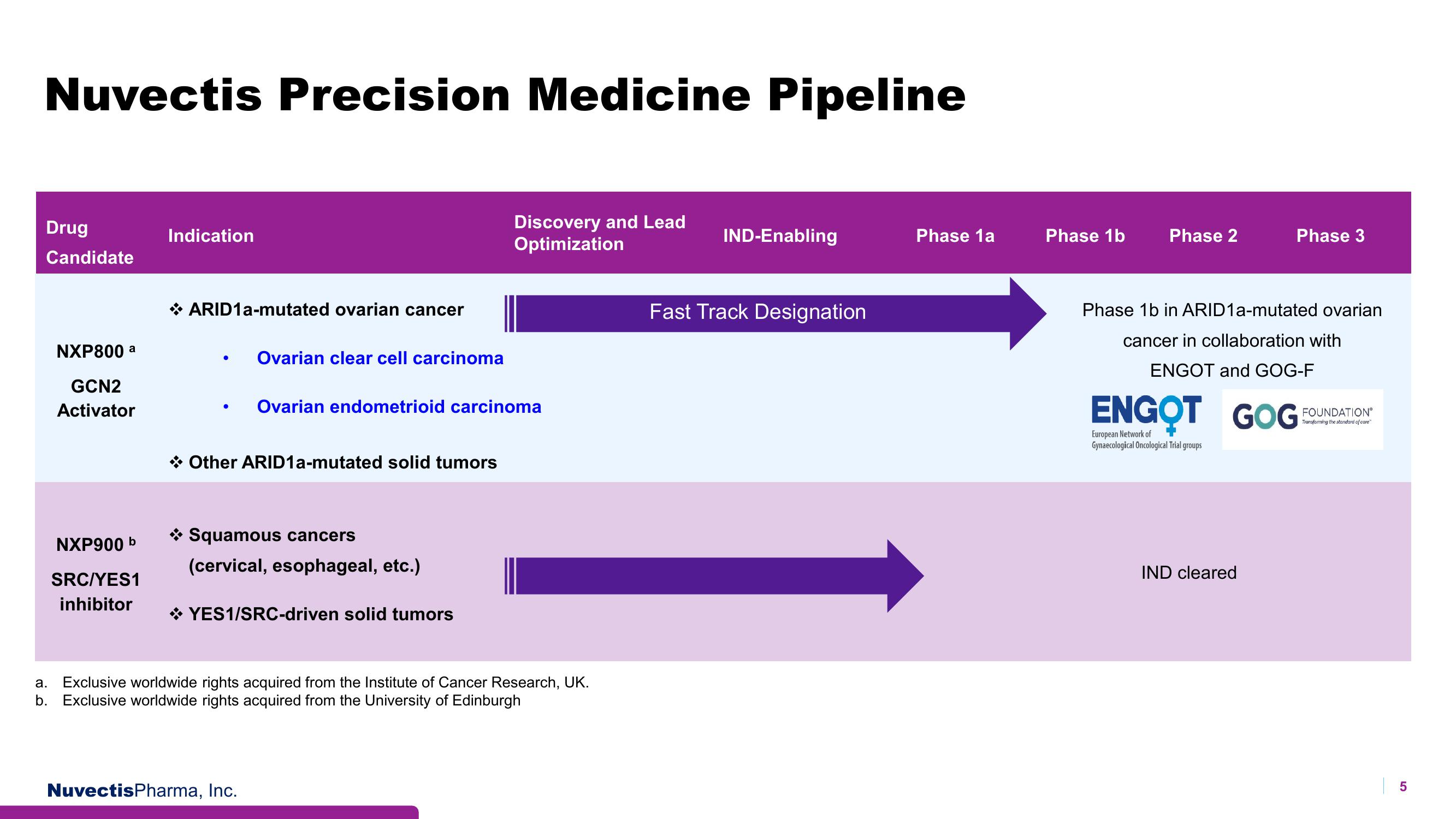
Nuvectis Precision Medicine Pipeline
Drug
Candidate
NXP800 a
GCN2
Activator
NXP900 b
SRC/YES1
inhibitor
Indication
ARID1a-mutated ovarian cancer
●
Ovarian clear cell carcinoma
→ Other ARID1a-mutated solid tumors
Ovarian endometrioid carcinoma
→ Squamous cancers
(cervical, esophageal, etc.)
YES1/SRC-driven solid tumors
NuvectisPharma, Inc.
Discovery and Lead
Optimization
a. Exclusive worldwide rights acquired from the Institute of Cancer Research, UK.
b. Exclusive worldwide rights acquired from the University of Edinburgh
IND-Enabling
Fast Track Designation
Phase 1a
Phase 1b
Phase 2
Phase 3
Phase 1b in ARID1a-mutated ovarian
cancer in collaboration with
ENGOT and GOG-F
ENGOT GOG
European Network of
Gynaecological Oncological Trial groups
IND cleared
FOUNDATION®
Transforming the standard of care
5View entire presentation