BioAtla Investor Conference Presentation Deck
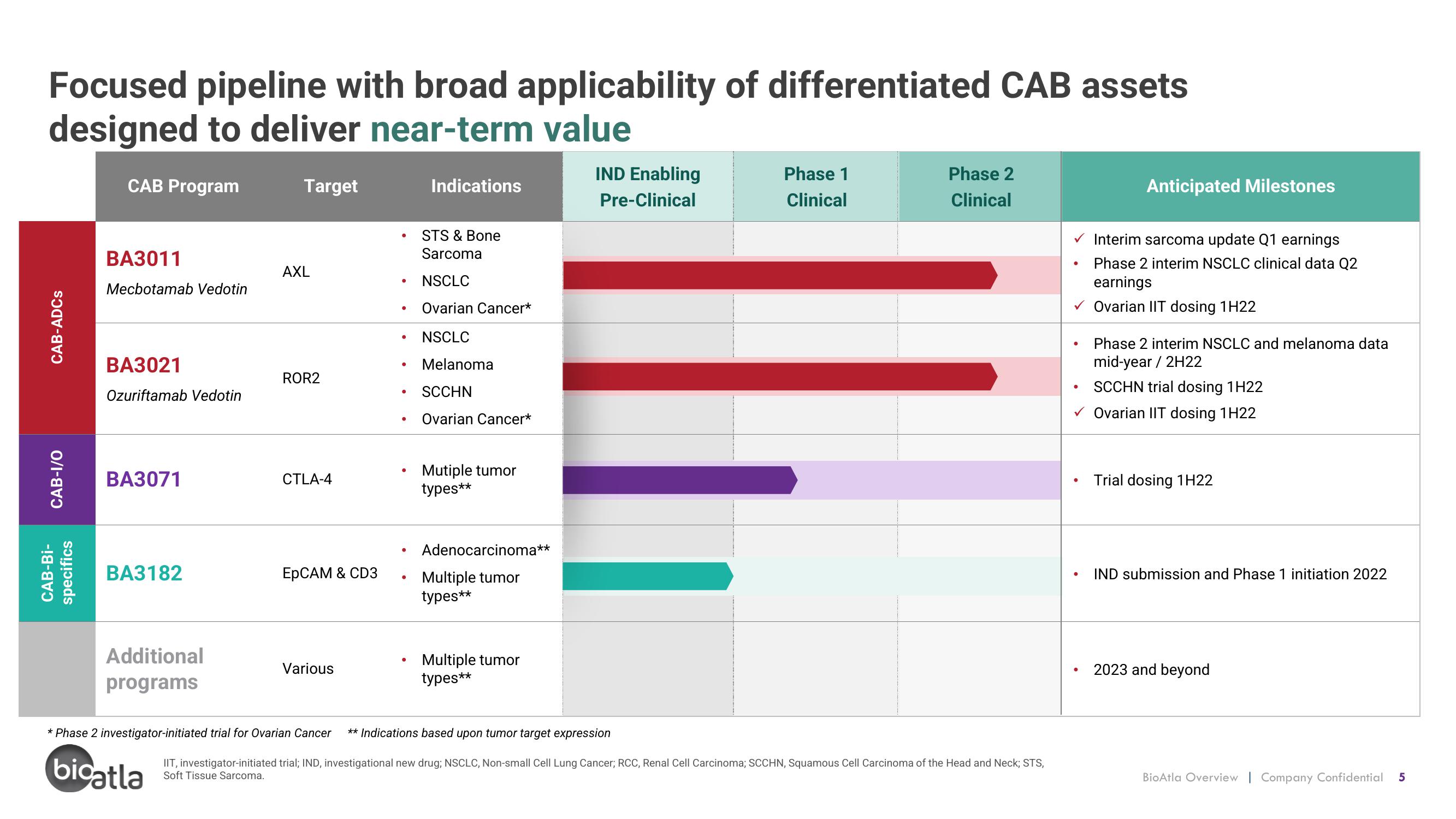
Focused pipeline with broad applicability of differentiated CAB assets
designed to deliver near-term value
CAB-ADCs
CAB-I/O
CAB-Bi-
specifics
CAB Program
BA3011
Mecbotamab Vedotin
BA3021
Ozuriftamab Vedotin
BA3071
BA3182
Additional
programs
Target
AXL
ROR2
CTLA-4
EpCAM & CD3
Various
●
●
●
●
Indications
STS & Bone
Sarcoma
NSCLC
Ovarian Cancer*
NSCLC
Melanoma
SCCHN
Ovarian Cancer*
Mutiple tumor
types**
Adenocarcinoma**
Multiple tumor
types**
Multiple tumor
types**
IND Enabling
Pre-Clinical
* Phase 2 investigator-initiated trial for Ovarian Cancer ** Indications based upon tumor target expression
bicatla
Phase 1
Clinical
Phase 2
Clinical
IIT, investigator-initiated trial; IND, investigational new drug; NSCLC, Non-small Cell Lung Cancer; RCC, Renal Cell Carcinoma; SCCHN, Squamous Cell Carcinoma of the Head and Neck; STS,
Soft Tissue Sarcoma.
Anticipated Milestones
✓ Interim sarcoma update Q1 earnings
Phase 2 interim NSCLC clinical data Q2
earnings
Ovarian IIT dosing 1H22
Phase 2 interim NSCLC and melanoma data
mid-year / 2H22
SCCHN trial dosing 1H22
✓ Ovarian IIT dosing 1H22
Trial dosing 1H22
IND submission and Phase 1 initiation 2022
2023 and beyond
BioAtla Overview | Company Confidential 5View entire presentation