Ocuphire Pharma Investor Day Presentation Deck
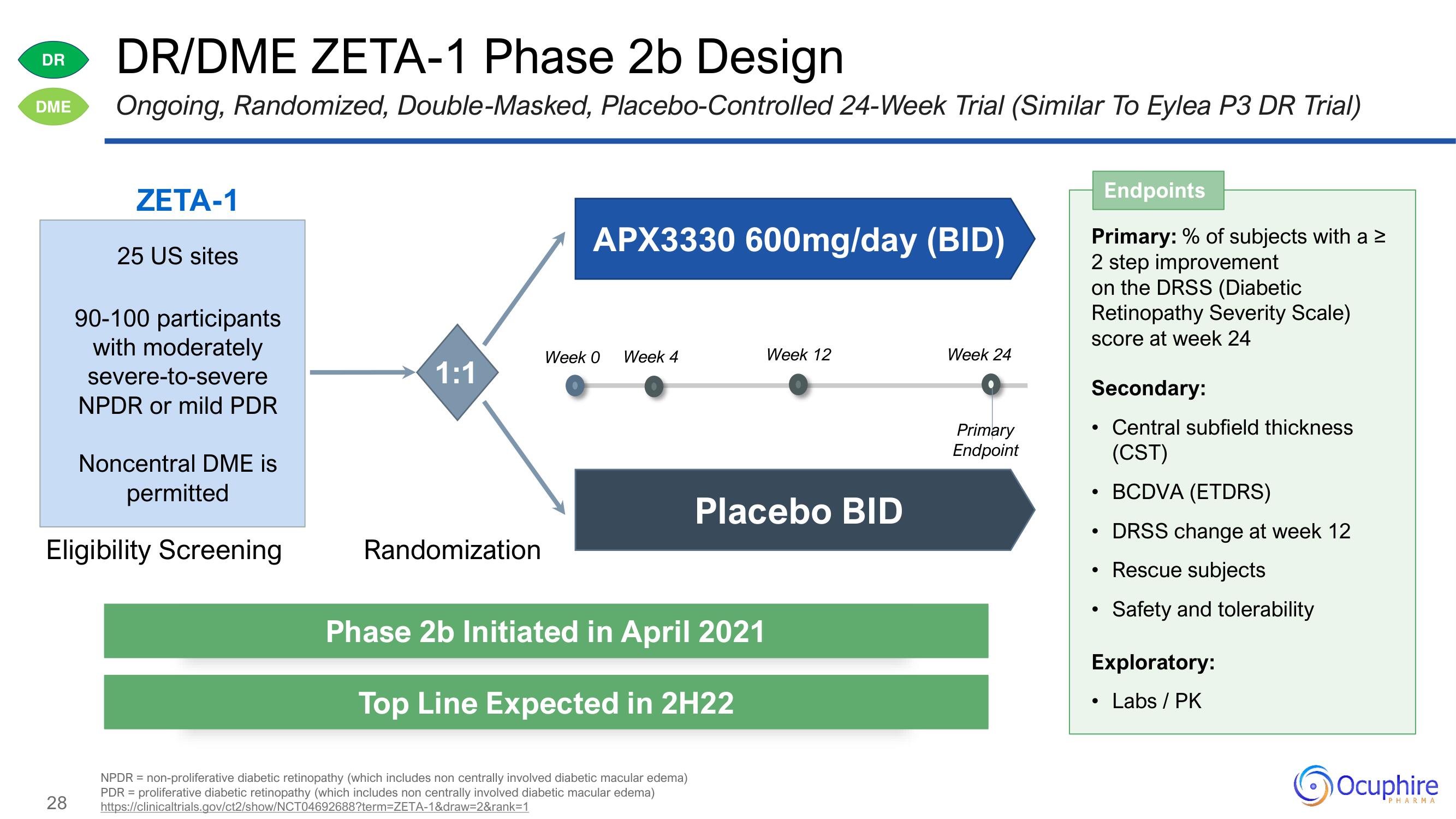
DR/DME ZETA-1 Phase 2b Design
DME Ongoing, Randomized, Double-Masked, Placebo-Controlled 24-Week Trial (Similar To Eylea P3 DR Trial)
DR
ZETA-1
28
25 US sites
90-100 participants
with moderately
severe-to-severe
NPDR or mild PDR
Noncentral DME is
permitted
Eligibility Screening
1:1
Randomization
APX3330 600mg/day (BID)
Week 0
Week 4
Phase 2b Initiated in April 2021
Top Line Expected in 2H22
NPDR = non-proliferative diabetic retinopathy (which includes non centrally involved diabetic macular edema)
PDR = proliferative diabetic retinopathy (which includes non centrally involved diabetic macular edema)
https://clinicaltrials.gov/ct2/show/NCT04692688?term=ZETA-1&draw=2&rank=1
Week 12
Placebo BID
Week 24
Primary
Endpoint
Endpoints
Primary: % of subjects with a 2
2 step improvement
on the DRSS (Diabetic
Retinopathy Severity Scale)
score at week 24
Secondary:
Central subfield thickness
(CST)
BCDVA (ETDRS)
●
●
DRSS change at week 12
• Rescue subjects
●
●
Safety and tolerability
Exploratory:
Labs / PK
●
Ocuphire
PHARMAView entire presentation