Aeglea BioTherapeutics Investor Presentation Deck
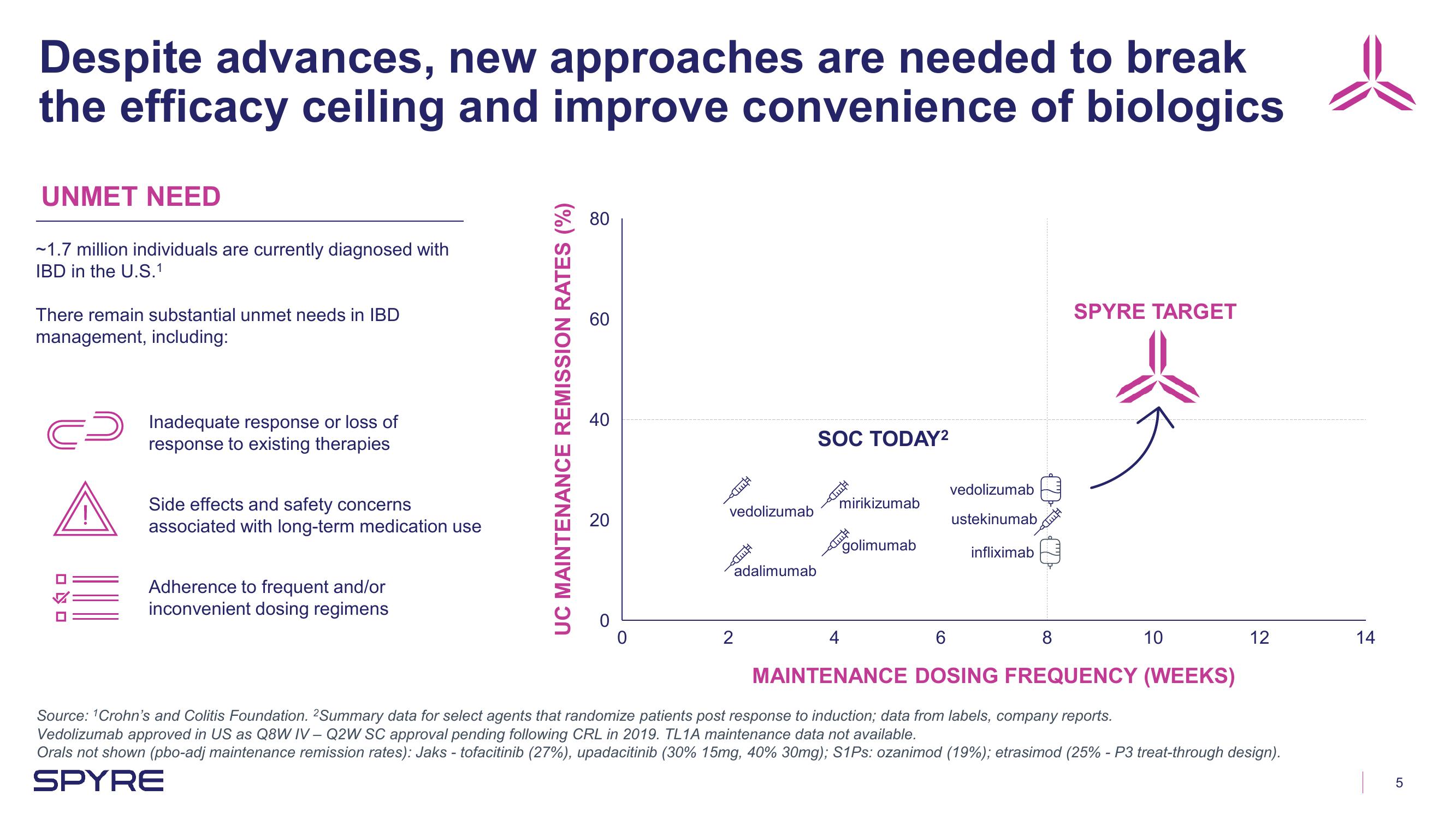
Despite advances, new approaches are needed to break
the efficacy ceiling and improve convenience of biologics
UNMET NEED
~1.7 million individuals are currently diagnosed with
IBD in the U.S.1
There remain substantial unmet needs in IBD
management, including:
co
OO
Inadequate response or loss of
response to existing therapies
Side effects and safety concerns
associated with long-term medication use
Adherence to frequent and/or
inconvenient dosing regimens
UC MAINTENANCE REMISSION RATES (%)
80
60
40
20
0
C
vedolizumab
buk
adalimumab
2
SOC TODAY²
mirikizumab
golimumab
vedolizumab
ustekinumab
infliximab
6
D
4
MAINTENANCE DOSING FREQUENCY (WEEKS)
SPYRE TARGET
8
10
12
Source: ¹Crohn's and Colitis Foundation. 2Summary data for select agents that randomize patients post response to induction; data from labels, company reports.
Vedolizumab approved in US as Q8W IV - Q2W SC approval pending following CRL in 2019. TL1A maintenance data not available.
Orals not shown (pbo-adj maintenance remission rates): Jaks - tofacitinib (27%), upadacitinib (30% 15mg, 40% 30mg); S1Ps: ozanimod (19%); etrasimod (25% - P3 treat-through design).
SPYRE
14
5View entire presentation