Bausch+Lomb Results Presentation Deck
NOV03¹: Investigational First in Class Treatment for Dry Eye Disease
Associated with Meibomian Gland Dysfunction
Decrease from Baseline (%)
Consistent statistically significant efficacy, safety and tolerability have now been demonstrated in two Phase 3 studies of NOV03¹ and one Phase 2 study
Studied in patients with dry eye disease associated with meibomian gland dysfunction
Statistically significant difference of sign and symptom was noted at day 15 and 57 in both Phase 3 studies
Anticipate filing NDA with the FDA in 1H22
N=311
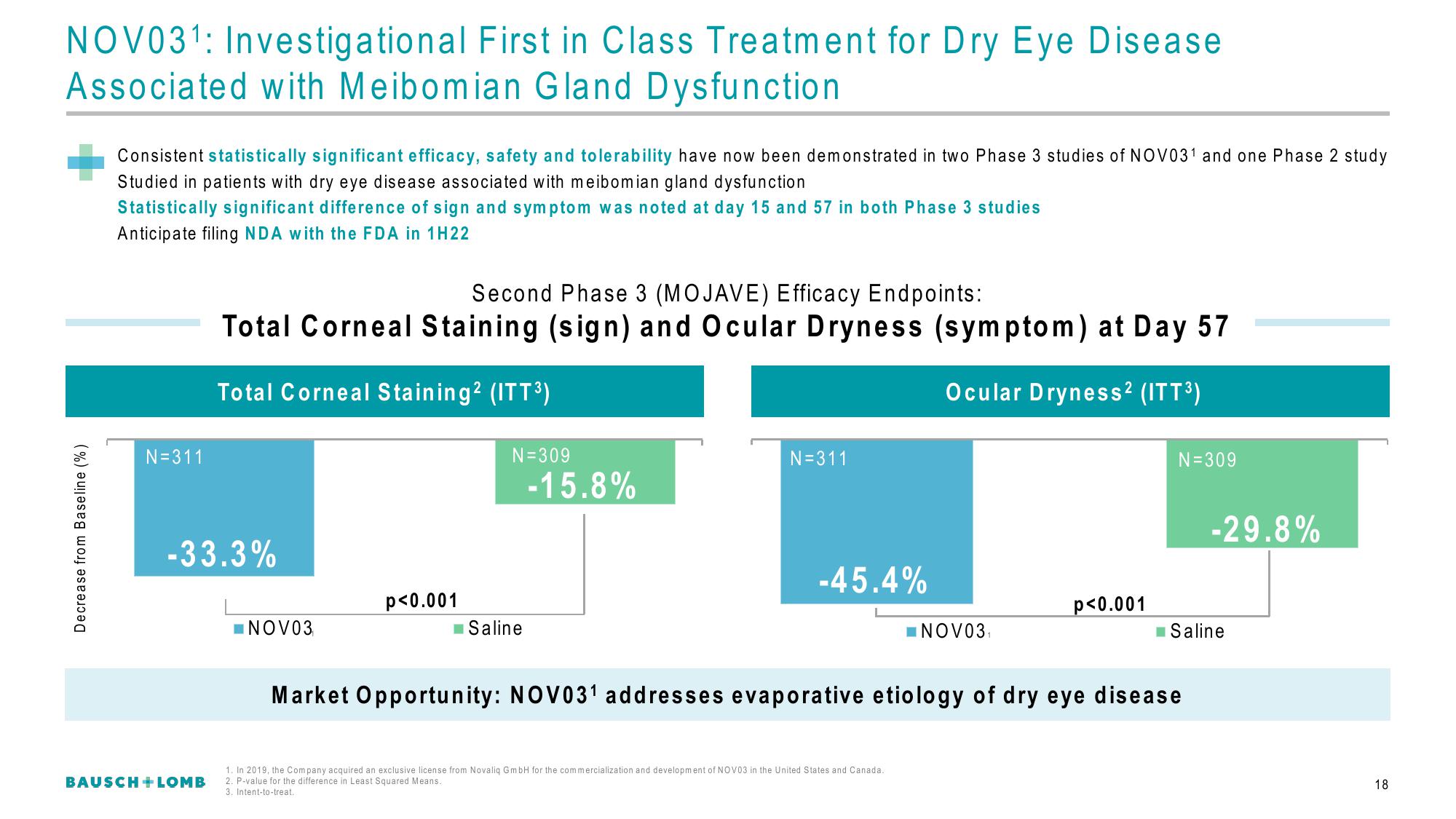
Second Phase 3 (MOJAVE) Efficacy Endpoints:
Total Corneal Staining (sign) and Ocular Dryness (symptom) at Day 57
Total Corneal Staining² (ITT³)
Ocular Dryness² (ITT³)
-33.3%
BAUSCH + LOMB
NOV03
p<0.001
N=309
Saline
-15.8%
N=311
-45.4%
■NOV03₁
1. In 2019, the Company acquired an exclusive license from Novaliq GmbH for the commercialization and development of NOV03 in the United States and Canada.
2. P-value for the difference in Least Squared Means.
3. Intent-to-treat.
p<0.001
N=309
Market Opportunity: NOV03¹ addresses evaporative etiology of dry eye disease
-29.8%
Saline
18View entire presentation