Taysha IPO Presentation Deck
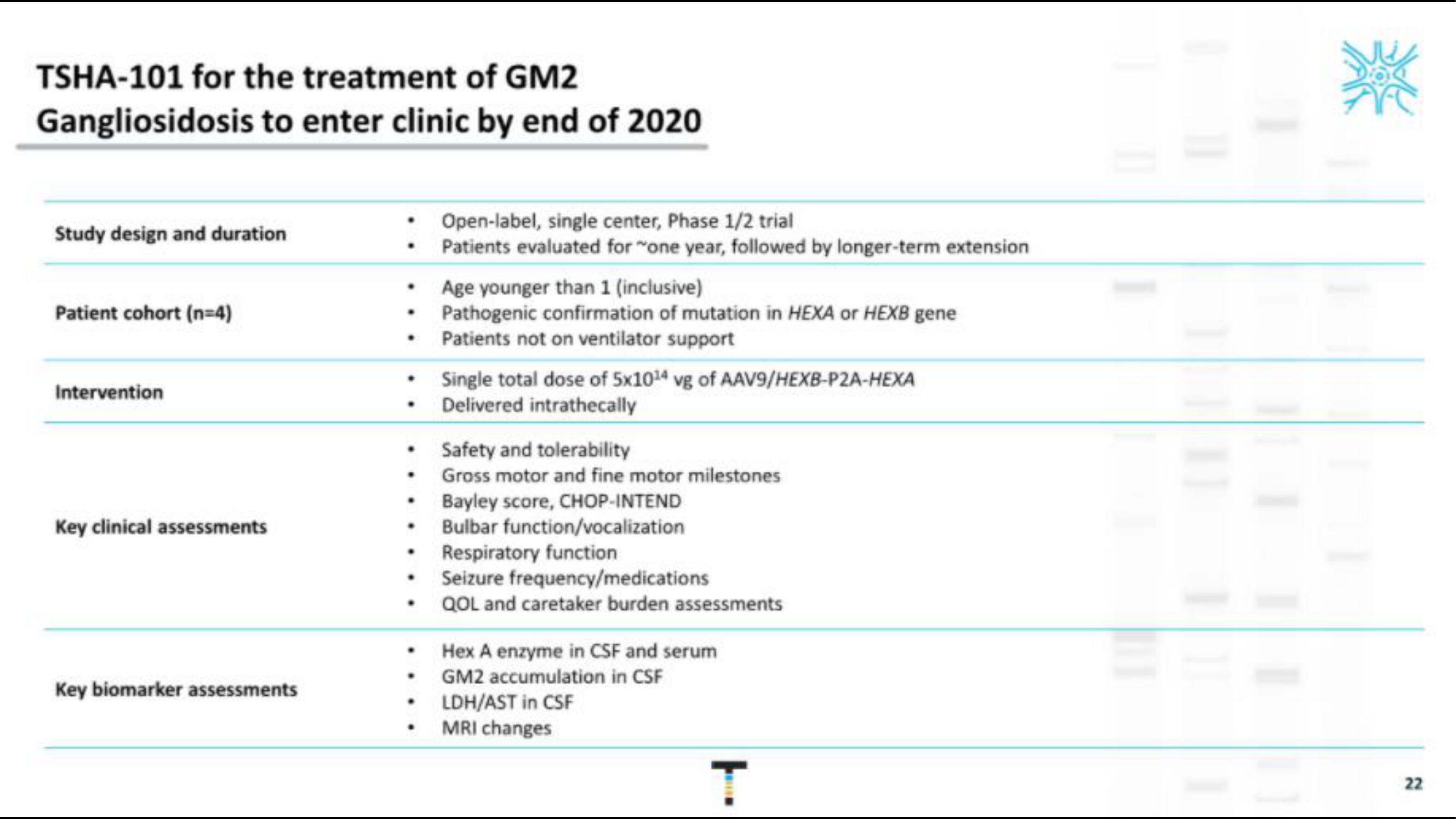
TSHA-101 for the treatment of GM2
Gangliosidosis to enter clinic by end of 2020
Study design and duration
Patient cohort (n=4)
Intervention
Key clinical assessments
Key biomarker assessments
.
.
•
•
.
Open-label, single center, Phase 1/2 trial
Patients evaluated for "one year, followed by longer-term extension
Age younger than 1 (inclusive)
Pathogenic confirmation of mutation in HEXA or HEXB gene
Patients not on ventilator support
Single total dose of 5x1014 vg of AAV9/HEXB-P2A-HEXA
Delivered intrathecally
Safety and tolerability
Gross motor and fine motor milestones
Bayley score, CHOP-INTEND
Bulbar function/vocalization
Respiratory function
Seizure frequency/medications
QOL and caretaker burden assessments
Hex A enzyme in CSF and serum
GM2 accumulation in CSF
LDH/AST in CSF
MRI changes
T
*
22View entire presentation