IDEAYA Biosciences Interim IDE397 Phase 1 Clinical Data and Q1 2022 Corporate Update
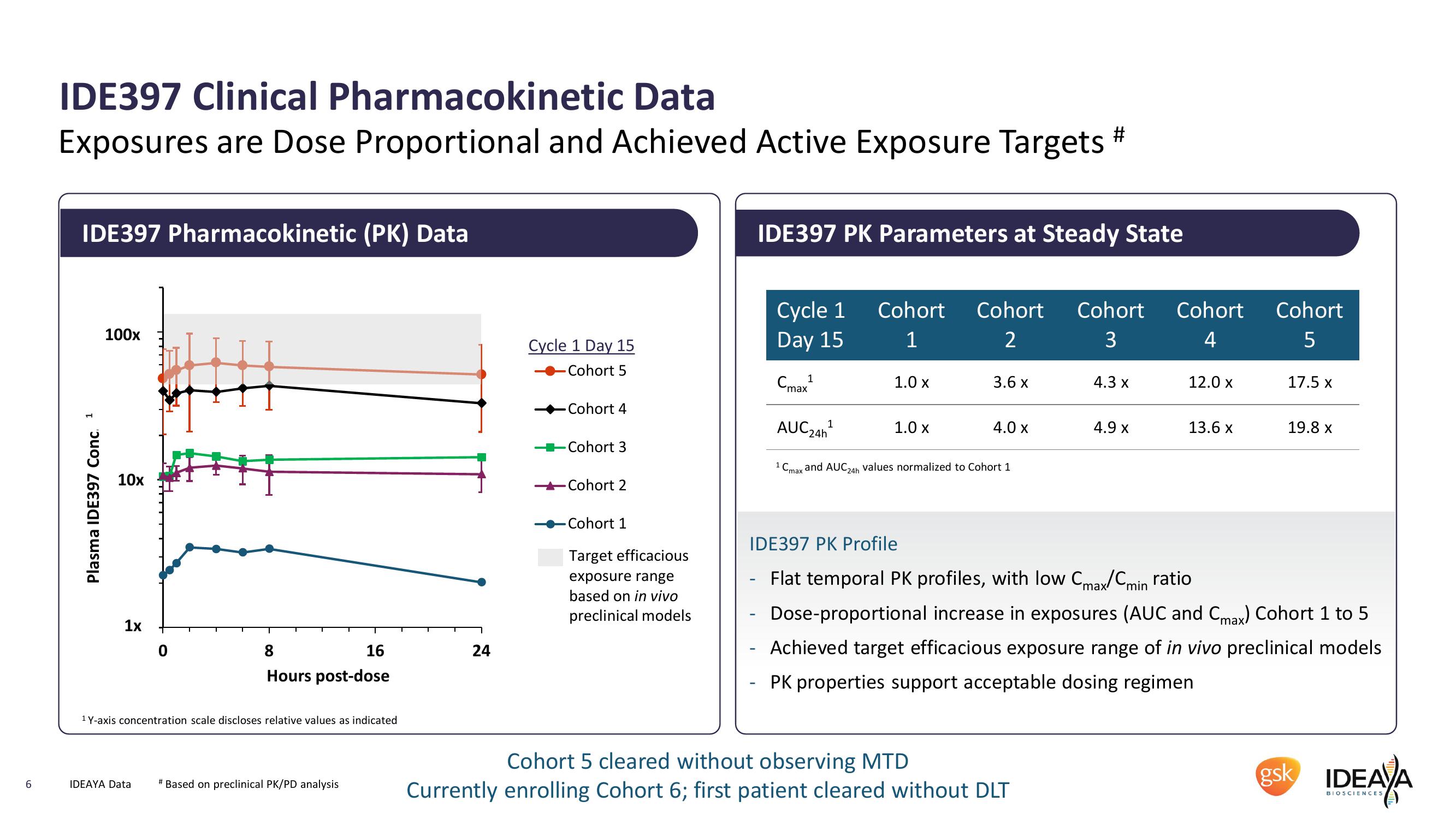
IDE397 Clinical Pharmacokinetic Data
Exposures are Dose Proportional and Achieved Active Exposure Targets #
IDE397 Pharmacokinetic (PK) Data
IDE397 PK Parameters at Steady State
Plasma IDE397 Conc. 1
100x
10x
1x
0
8
16
24
Hours post-dose
1 Y-axis concentration scale discloses relative values as indicated
Cycle 1 Day 15
Cohort 5
Cycle 1
Day 15
Cohort Cohort Cohort
Cohort
Cohort
1
2
3
4
5
Стах 1
1.0 x
3.6 x
4.3 x
12.0 x
17.5 x
max
Cohort 4
AUC 24h¹
1.0 x
4.0 x
4.9 x
13.6 x
19.8 x
-Cohort 3
max
1 C. and AUC 24h values normalized to Cohort 1
-Cohort 2
-Cohort 1
Target efficacious
exposure range
based on in vivo
preclinical models
-
-
IDE397 PK Profile
Flat temporal PK profiles, with low Cmax/Cmin ratio
Dose-proportional increase in exposures (AUC and Cmax) Cohort 1 to 5
Achieved target efficacious exposure range of in vivo preclinical models
PK properties support acceptable dosing regimen
Cohort 5 cleared without observing MTD
6
IDEAYA Data
#Based on preclinical PK/PD analysis
Currently enrolling Cohort 6; first patient cleared without DLT
gsk
IDEA A
BIOSCIENCESView entire presentation