BioNTech Investor Presentation Deck
Oncology Programs in Phase 2
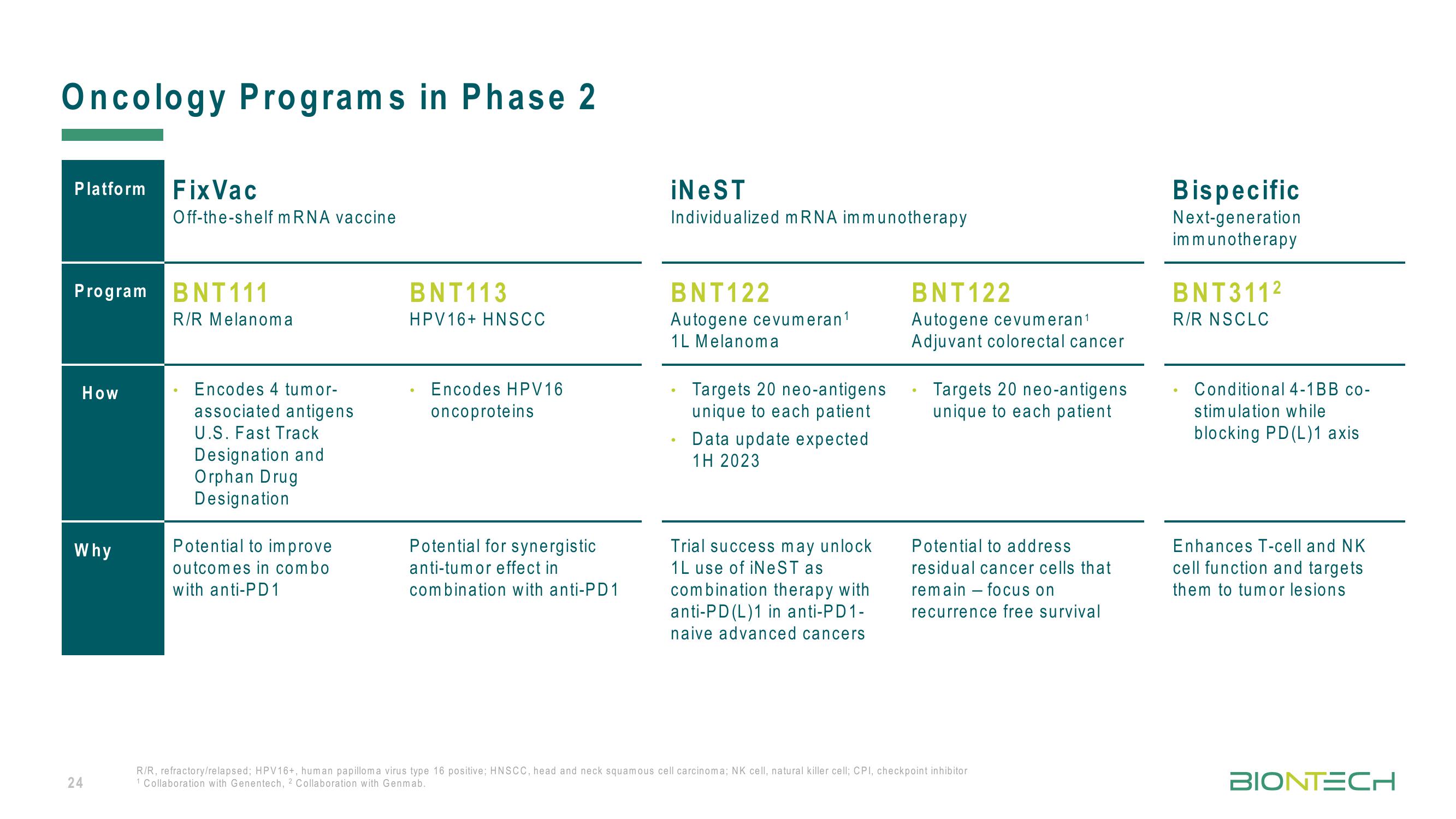
Platform Fix Vac
Program BNT111
How
Why
Off-the-shelf mRNA vaccine
24
R/R Melanoma
Encodes 4 tumor-
associated antigens
U.S. Fast Track
Designation and
Orphan Drug
Designation
Potential to improve
outcomes in combo
with anti-PD1
BNT113
HPV16+ HNSCC
Encodes HPV16
oncoproteins
Potential for synergistic
anti-tumor effect in
combination with anti-PD1
iNeST
Individualized mRNA immunotherapy
BNT122
Autogene cevumeran¹
1L Melanoma
Targets 20 neo-antigens
unique to each patient
Data update expected
1H 2023
Trial success may unlock
1L use of iNeST as
combination therapy with
anti-PD (L)1 in anti-PD1-
naive advanced cancers
BNT122
Autogene cevumeran¹
Adjuvant colorectal cancer
Targets 20 neo-antigens
unique to each patient
Potential to address
residual cancer cells that
remain focus on
recurrence free survival
R/R, refractory/relapsed; HPV16+, human papilloma virus type 16 positive; HNSCC, head and neck squamous cell carcinoma; NK cell, natural killer cell; CPI, checkpoint inhibitor
1 Collaboration with Genentech, 2 Collaboration with Genmab.
Bispecific
Next-generation
immunotherapy
BNT311²
R/R NSCLC
Conditional 4-1BB co-
stimulation while
blocking PD(L)1 axis
Enhances T-cell and NK
cell function and targets
them to tumor lesions
BIONTECHView entire presentation