23andMe Investor Day Presentation Deck
GSK6097608: Phase 1 Study Design
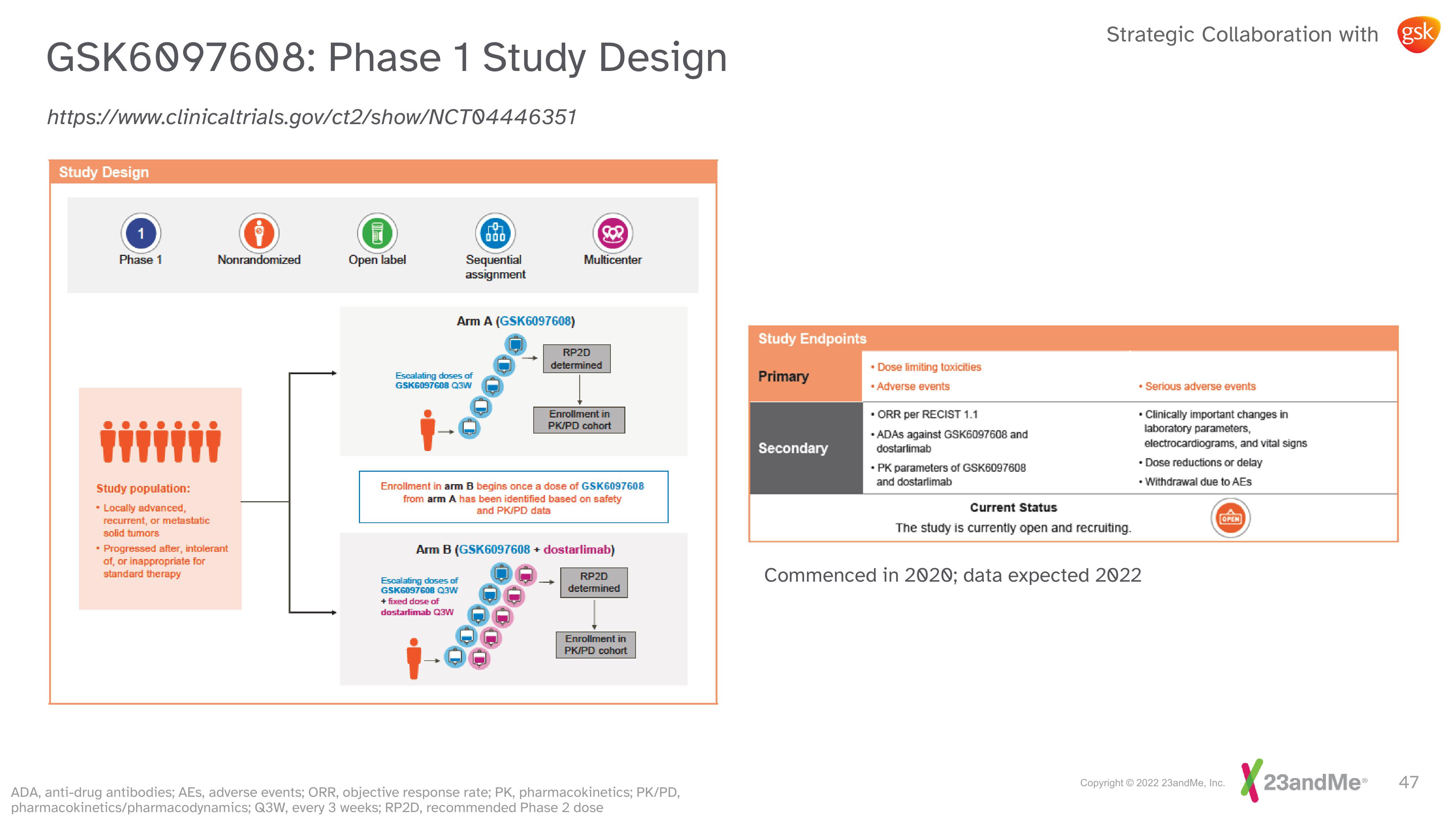
https://www.clinical trials.gov/ct2/show/NCT04446351
Study Design
1
Phase 1
Study population:
• Locally advanced,
recurrent, or metastatic
solid tumors
Nonrandomized
• Progressed after, intolerant
of, or inappropriate for
standard therapy
Open label
Sequential
assignment
Arm A (GSK6097608)
Escalating doses of
GSK6097608 Q3W
0₂
000
L
Escalating doses of
GSK6097608 Q3W
+ fixed dose of
dostarlimab Q3W
Multicenter
RP2D
determined
Enrollment in
PK/PD cohort
Enrollment in arm B begins once a dose of GSK6097608
from arm A has been identified based on safety
and PK/PD data
Arm B (GSK6097608 + dostarlimab)
RP2D
determined
Enrollment in
PK/PD cohort
ADA, anti-drug antibodies; AEs, adverse events; ORR, objective response rate; PK, pharmacokinetics; PK/PD,
pharmacokinetics/pharmacodynamics; Q3W, every 3 weeks; RP2D, recommended Phase 2 dose
Study Endpoints
Primary
Secondary
• Dose limiting toxicities
• Adverse events
• ORR per RECIST 1.1
•ADAS against GSK6097608 and
dostarlimab
• PK parameters of GSK6097608
and dostarlimab
Strategic Collaboration with gsk
Current Status
The study is currently open and recruiting.
. Serious adverse events
. Clinically important changes in
laboratory parameters,
electrocardiograms, and vital signs
• Dose reductions or delay
• Withdrawal due to AEs
Commenced in 2020; data expected 2022
A
OPEN
Copyright © 2022 23andMe, Inc.
23andMe®
47View entire presentation