AstraZeneca Results Presentation Deck
Breast cancer
Progressing pipeline across multiple modalities
Lynparza adjuvant breast cancer
Phase III OlympiA trial unblinded
IDMC¹ recommended trial move.
to primary analysis and reporting
based on planned interim
analysis of primary endpoint
iDFS²
Anticipated to become new
standard of care in the treatment
of BRCAm high-risk HER2-
negative early breast cancer
First PARPI to demonstrate benefit
in BRCAm adjuvant breast cancer
1. Independent Data Monitoring Committee 2. Invasive disease-free survival.
32
PARP1-selective inhibitor
Five abstracts at AACR³
Selective PARP1-DNA trapper
More potent and efficacious than
first-generation PARP inhibitors
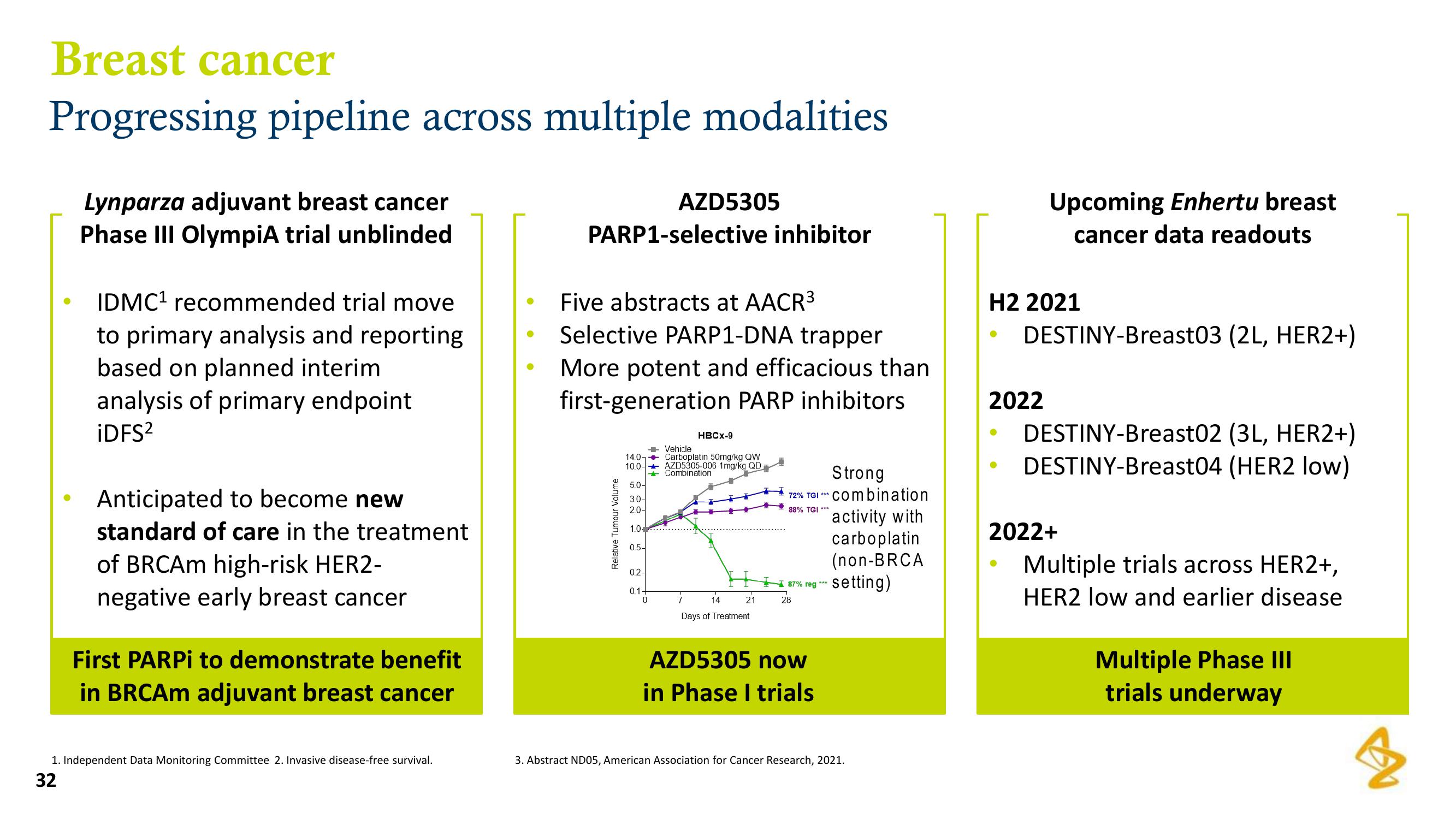
Relative Tumour Volume
14.0
10.0
5.0-
30-
2.0-
1.0
0.5-
0.2-
AZD5305
01.
→ Vehicle
0
HBCX-9
Carboplatin 50mg/kg QW
AZD5305-006 1mg/kg QD
Combination
14
21
Days of Treatment
Strong
72% TGI Combination
88% TGI ***
87% reg ***
28
activity with
carboplatin
(non-BRCA
-setting)
AZD5305 now
in Phase I trials
3. Abstract ND05, American Association for Cancer Research, 2021.
Upcoming Enhertu breast
cancer data readouts
H2 2021
DESTINY-Breast03 (2L, HER2+)
2022
DESTINY-Breast02 (3L, HER2+)
DESTINY-Breast04 (HER2 low)
2022+
Multiple trials across HER2+,
HER2 low and earlier disease
Multiple Phase III
trials underway
3View entire presentation