Ocuphire Pharma Results
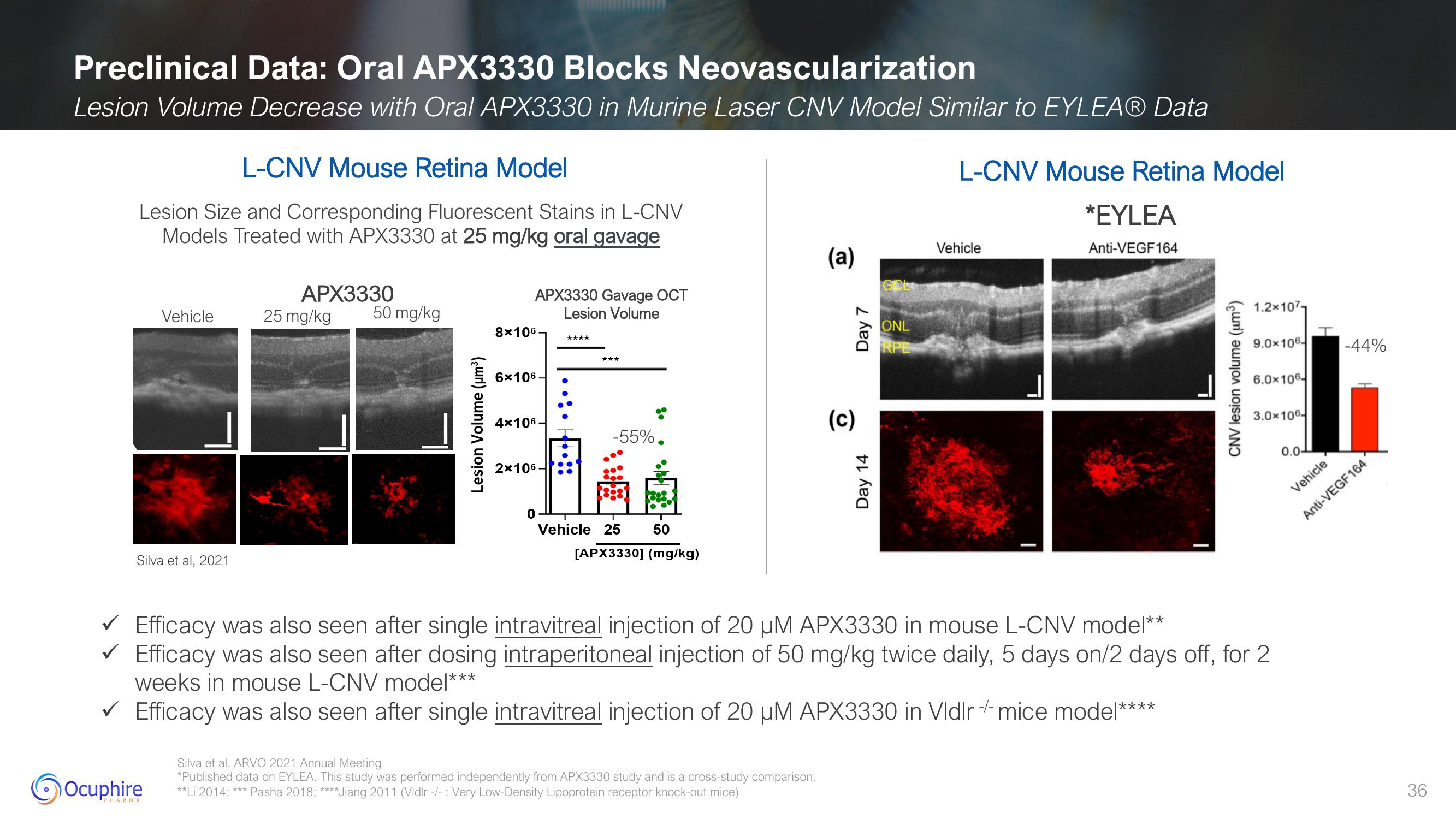
Preclinical Data: Oral APX3330 Blocks Neovascularization
Lesion Volume Decrease with Oral APX3330 in Murine Laser CNV Model Similar to EYLEAⓇ Data
L-CNV Mouse Retina Model
Lesion Size and Corresponding Fluorescent Stains in L-CNV
Models Treated with APX3330 at 25 mg/kg oral gavage
Silva et al, 2021
Vehicle
Ocuphire
PHARMA
APX3330
25 mg/kg
50 mg/kg
Lesion Volume (µm³)
APX3330 Gavage OCT
Lesion Volume
8x106
6x106
4x106-
2x106-
0
****
***
-55%.
Vehicle 25
50
[APX3330] (mg/kg)
(a)
Silva et al. ARVO 2021 Annual Meeting
*Published data on EYLEA. This study was performed independently from APX3330 study and is a cross-study comparison.
**Li 2014; *** Pasha 2018; **** *Jiang 2011 (Vldlr -/- Very Low-Density Lipoprotein receptor knock-out mice)
Day 7
(c)
Day 14
ONL
RPE
L-CNV Mouse Retina Model
Vehicle
*EYLEA
Anti-VEGF164
CNV lesion volume (um³)
1.2x107-
9.0x106- -44%
✓ Efficacy was also seen after single intravitreal injection of 20 µM APX3330 in mouse L-CNV model**
✓ Efficacy was also seen after dosing intraperitoneal injection of 50 mg/kg twice daily, 5 days on/2 days off, for 2
weeks in mouse L-CNV model**
***
✓ Efficacy was also seen after single intravitreal injection of 20 µM APX3330 in Vldlr / mice model*
6.0x106-
3.0x106-
0.0
Vehicle
Anti-VEGF164
36View entire presentation