ATAI Investor Presentation Deck
SUMMARY
OWNERSHIP 53.8%¹
PRODUCT
PHARMA-
COLOGY
PRODUCT
FEATURES
INDICATIONS
CURRENT
STATUS
INTELLECTUAL
PROPERTY
HIGHLIGHT
Deuterated etifoxine HCI oral dosage form
(GRX-917)
Etifoxine facilitates endogenous production of
neurosteroids like allopregnanolone through
agonist activity at the mitochondrial
translocator protein (TSPO)
GRX-917 is designed to have rapid onset activity
of anxiolytic activity like benzodiazepines but
without the sedating, addicting, or cognitive
impairing properties
Primary: Generalized Anxiety Disorder
Potential: Social Anxiety Disorder,
Postpartum Depression
Phase 1 trial initiated in H1'21
Issued composition of matter on deuterated
etifoxine (GRX-917) and corresponding
methods of use
GRX-917 is aimed to be an improved version of
Etifoxine, which already showed promising
results
28
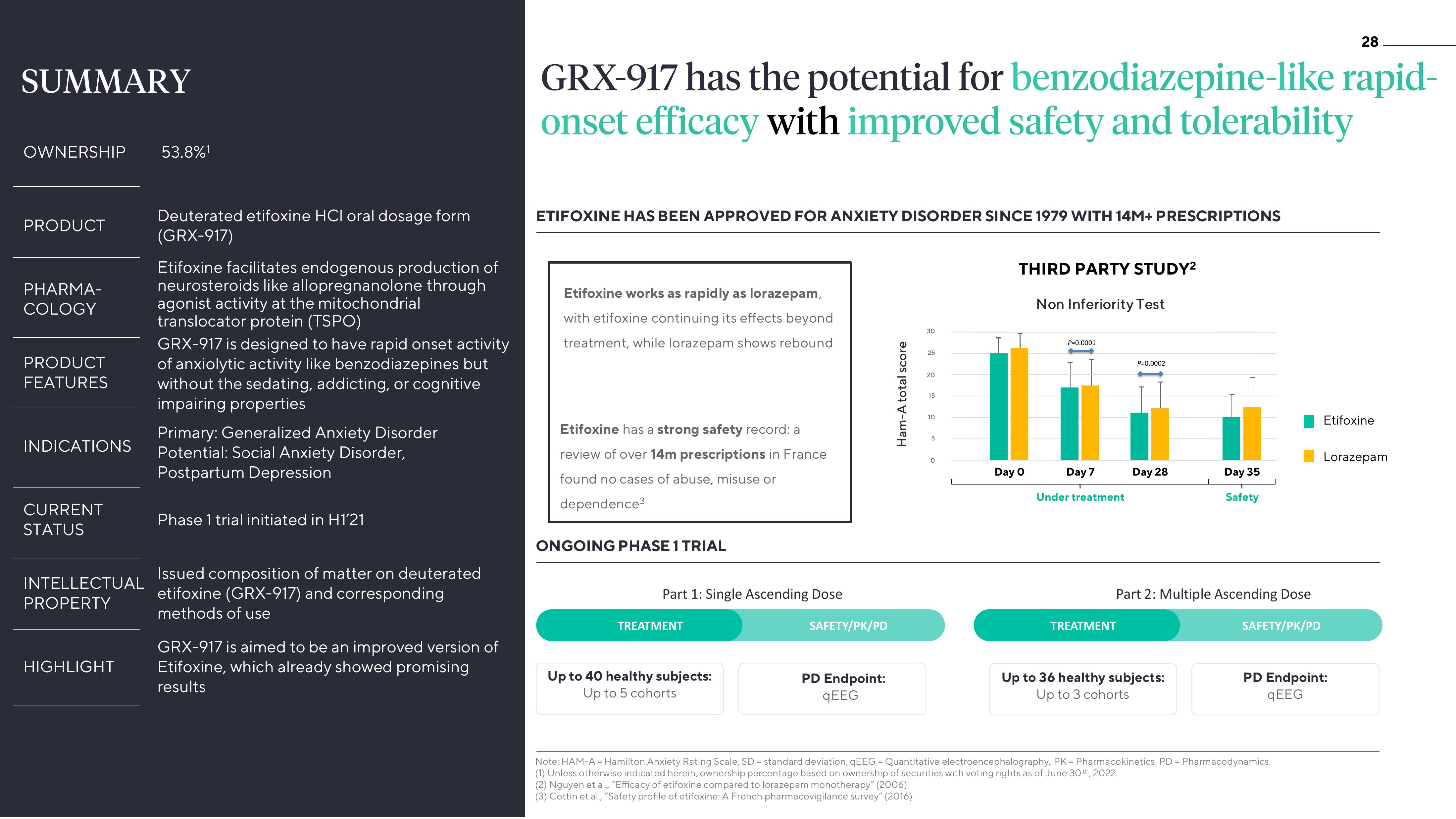
GRX-917 has the potential for benzodiazepine-like rapid-
onset efficacy with improved safety and tolerability
ETIFOXINE HAS BEEN APPROVED FOR ANXIETY DISORDER SINCE 1979 WITH 14M+ PRESCRIPTIONS
Etifoxine works as rapidly as lorazepam,
with etifoxine continuing its effects beyond
treatment, while lorazepam shows rebound
Etifoxine has a strong safety record: a
review of over 14m prescriptions in France
found no cases of abuse, misuse or
dependence³
ONGOING PHASE 1 TRIAL
Part 1: Single Ascending Dose
TREATMENT
Up to 40 healthy subjects:
Up to 5 cohorts
SAFETY/PK/PD
PD Endpoint:
qEEG
Ham-A total score
30
(2) Nguyen et al., "Efficacy of etifoxine compared to lorazepam monotherapy" (2006)
(3) Cottin et al., "Safety profile of etifoxine: A French pharmacovigilance survey" (2016)
20
15
5
0
THIRD PARTY STUDY²
Non Inferiority Test
Day 0
P=0.0001
Day 7
Under treatment
P=0.0002
TREATMENT
Day 28
Part 2: Multiple Ascending Dose
Day 35
Safety
Up to 36 healthy subjects:
Up to 3 cohorts
SAFETY/PK/PD
Note: HAM-A = Hamilton Anxiety Rating Scale, SD = standard deviation, qEEG = Quantitative electroencephalography, PK = Pharmacokinetics. PD = Pharmacodynamics.
(1) Unless otherwise indicated herein, ownership percentage based on ownership of securities with voting rights as of June 30th, 2022.
Etifoxine
Lorazepam
PD Endpoint:
qEEGView entire presentation