Ocuphire Pharma Investor Day Presentation Deck
DR
DME
26
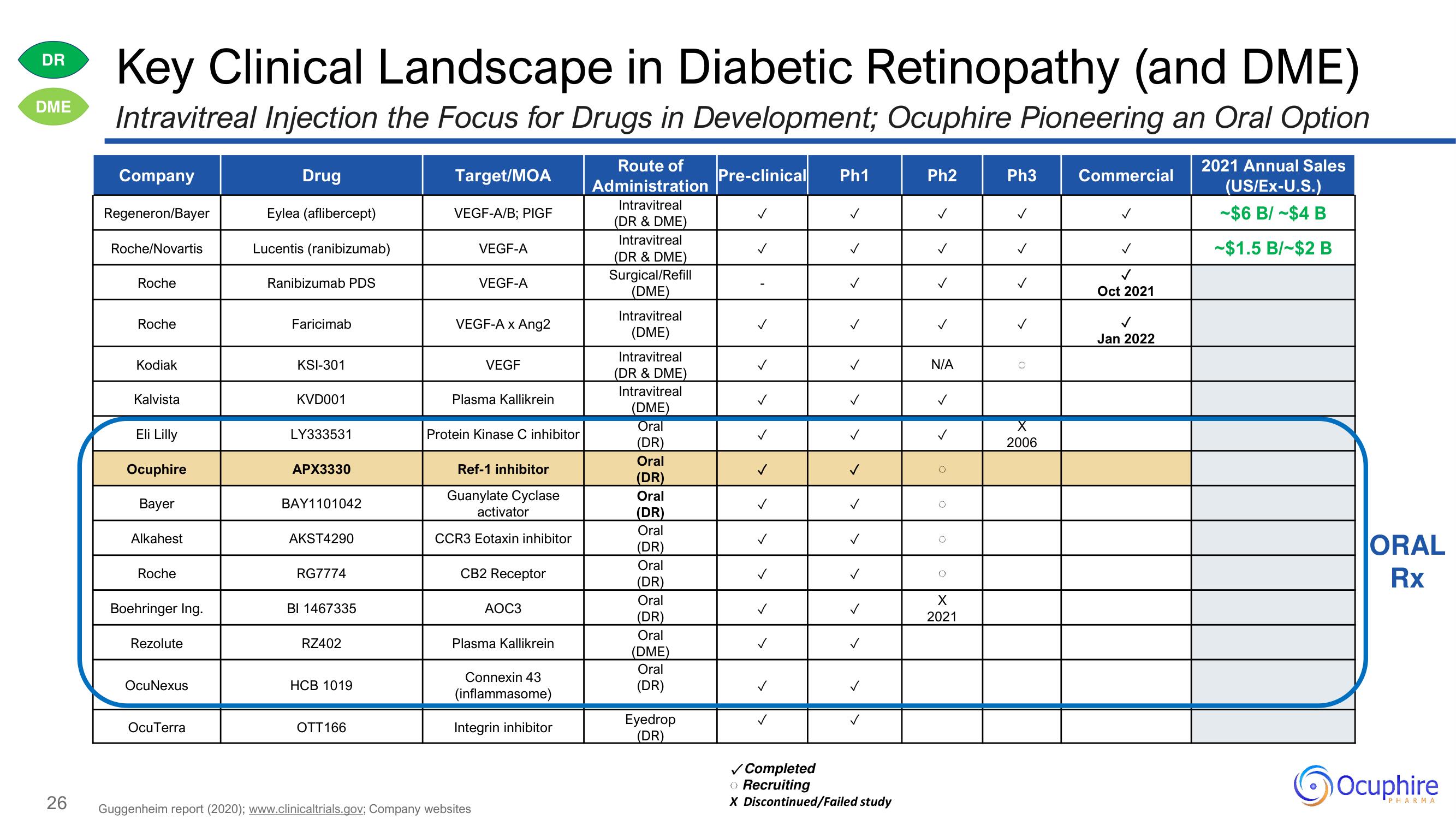
Key Clinical Landscape in Diabetic Retinopathy (and DME)
Intravitreal Injection the Focus for Drugs in Development; Ocuphire Pioneering an Oral Option
Company
Regeneron/Bayer
Roche/Novartis
Roche
Roche
Kodiak
Kalvista
Eli Lilly
Ocuphire
Bayer
Alkahest
Roche
Boehringer Ing.
Rezolute
OcuNexus
OcuTerra
Drug
Eylea (aflibercept)
Lucentis (ranibizumab)
Ranibizumab PDS
Faricimab
KSI-301
KVD001
LY333531
APX3330
BAY1101042
AKST4290
RG7774
BI 1467335
RZ402
HCB 1019
OTT 166
Target/MOA
VEGF-A/B; PIGF
VEGF-A
VEGF-A
VEGF-A x Ang2
VEGF
Plasma Kallikrein
Protein Kinase C inhibitor
Ref-1 inhibitor
Guanylate Cyclase
activator
CCR3 Eotaxin inhibitor
CB2 Receptor
Guggenheim report (2020); www.clinicaltrials.gov; Company websites
AOC3
Plasma Kallikrein
Connexin 43
(inflammasome)
Integrin inhibitor
Route of
Administration
Intravitreal
(DR & DME)
Intravitreal
(DR & DME)
Surgical/Refill
(DME)
Intravitreal
(DME)
Intravitreal
(DR & DME)
Intravitreal
(DME)
Oral
(DR)
Oral
(DR)
Oral
(DR)
Oral
(DR)
Oral
(DR)
Oral
(DR)
Oral
(DME)
Oral
(DR)
Eyedrop
(DR)
Pre-clinical Ph1
✓
✓
✓
✓
✓
✓
✓
✓
✓
✓
✓
✓
✓ Completed
O Recruiting
X Discontinued/Failed study
Ph2
✓
✓
✓
N/A
✓
✓
0
X
2021
Ph3
✓
✓
✓
✓
X
2006
Commercial
✓
✓
Oct 2021
Jan 2022
2021 Annual Sales
(US/EX-U.S.)
-$6 B/-$4 B
-$1.5 B/-$2 B
ORAL
Rx
Ocuphire
PHARMAView entire presentation