Ocuphire Pharma Results Presentation Deck
RM
8
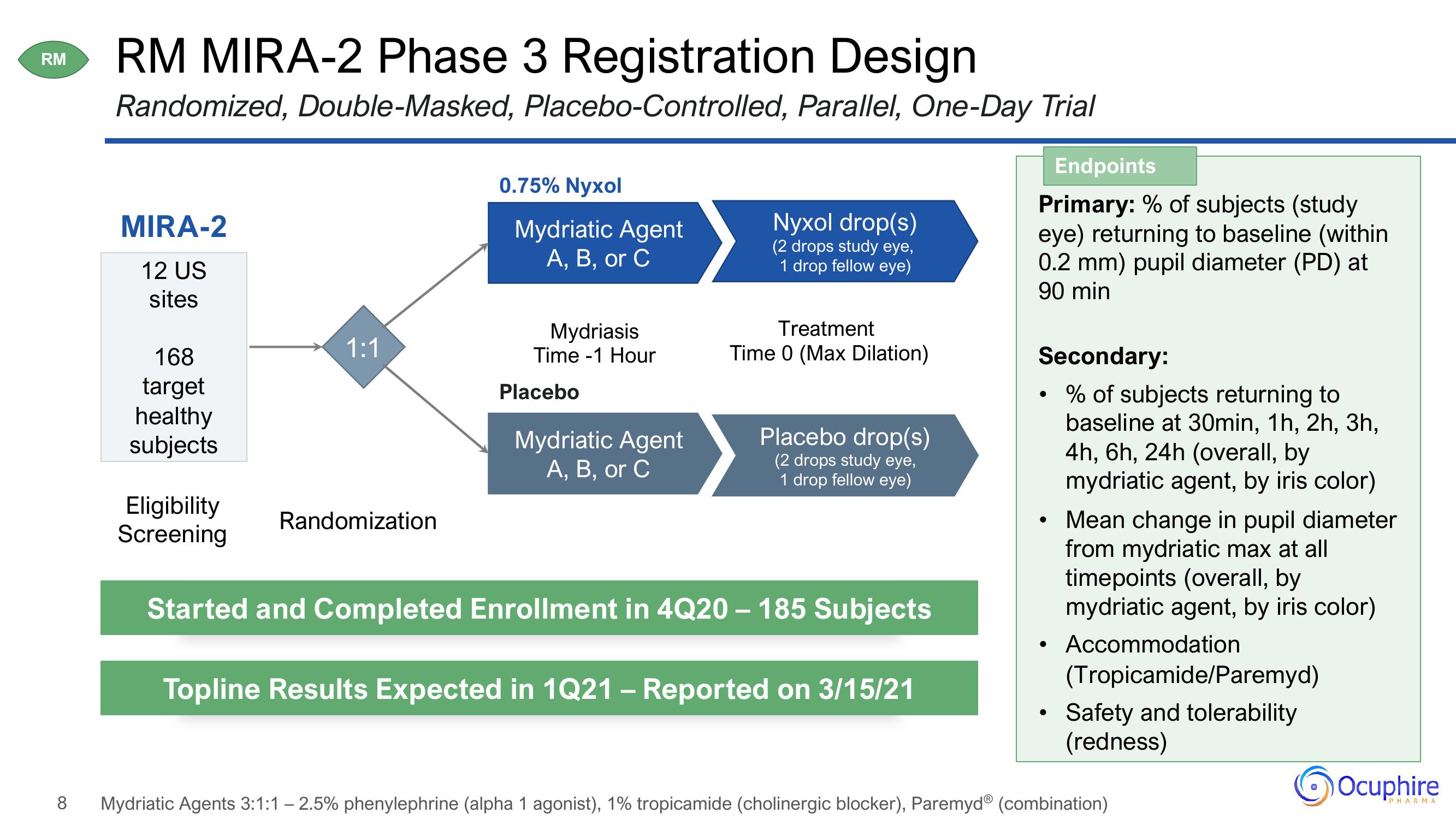
RM MIRA-2 Phase 3 Registration Design
Randomized, Double-Masked, Placebo-Controlled, Parallel, One-Day Trial
MIRA-2
12 US
sites
168
target
healthy
subjects
Eligibility
Screening
1:1
Randomization
0.75% Nyxol
Mydriatic Agent
A, B, or C
Mydriasis
Time 1 Hour
Placebo
Mydriatic Agent
A, B, or C
Nyxol drop(s)
(2 drops study eye,
1 drop fellow eye)
Treatment
Time 0 (Max Dilation)
Placebo drop(s)
(2 drops study eye,
1 drop fellow eye)
Started and Completed Enrollment in 4Q20 - 185 Subjects
Topline Results Expected in 1Q21 – Reported on 3/15/21
Endpoints
Primary: % of subjects (study
eye) returning to baseline (within
0.2 mm) pupil diameter (PD) at
90 min
Secondary:
• % of subjects returning to
baseline at 30min, 1h, 2h, 3h,
4h, 6h, 24h (overall, by
mydriatic agent, by iris color)
●
●
●
Mean change in pupil diameter
from mydriatic max at all
timepoints (overall, by
mydriatic agent, by iris color)
Accommodation
(Tropicamide/Paremyd)
Safety and tolerability
(redness)
Mydriatic Agents 3:1:1 - 2.5% phenylephrine (alpha 1 agonist), 1% tropicamide (cholinergic blocker), Paremyd® (combination)
Ocuphire
PHARMAView entire presentation