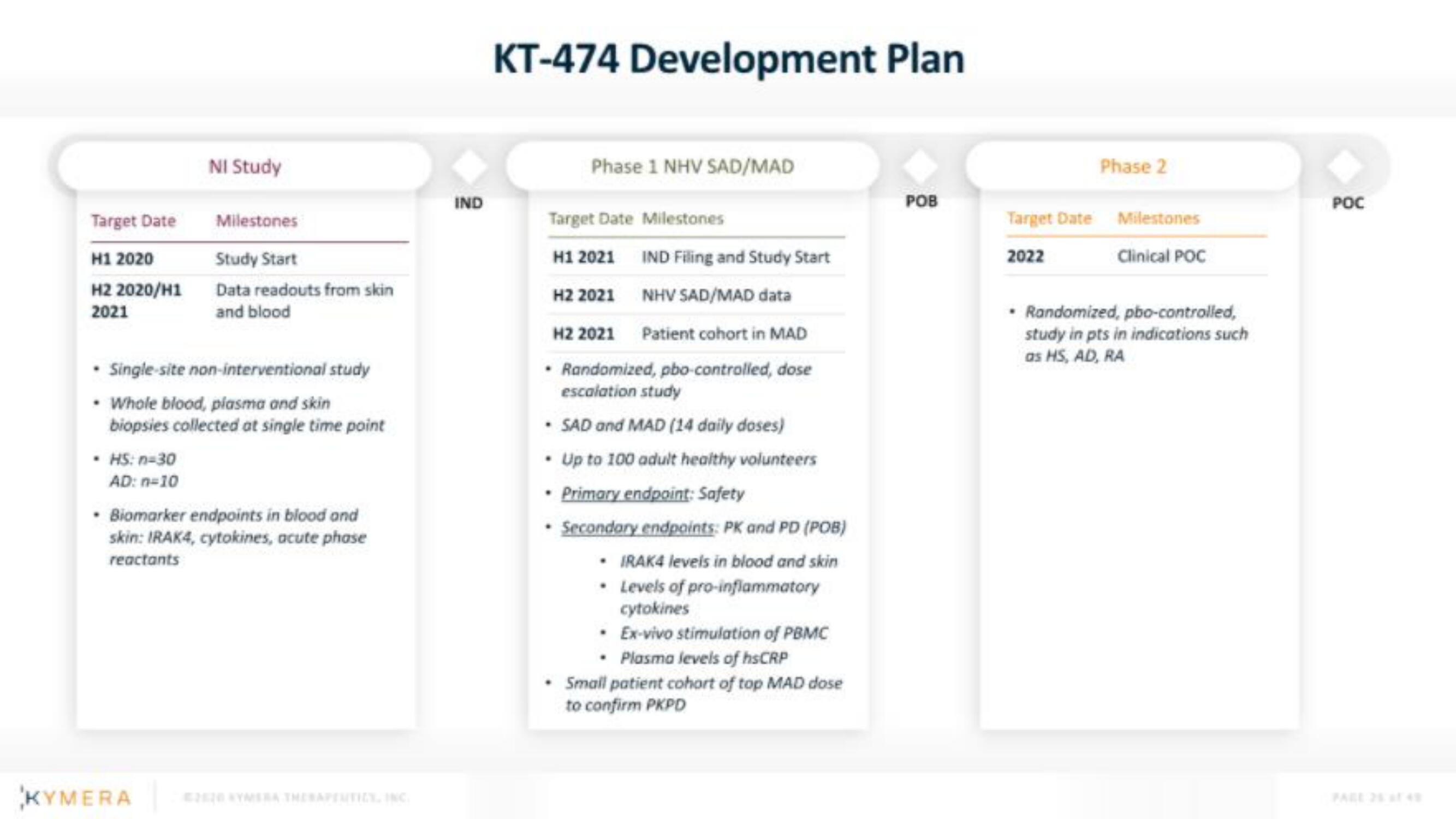
Kymera IPO Presentation Deck
Target Date
H1 2020
H2 2020/H1
2021
• HS: n=30
AD: n=10
NI Study
Single-site non-interventional study
• Whole blood, plasma and skin
biopsies collected at single time point
Milestones
Study Start
Data readouts from skin
and blood
KYMERA
• Biomarker endpoints in blood and
skin: IRAK4, cytokines, acute phase
reactants
IND
KT-474 Development Plan
Phase 1 NHV SAD/MAD
Target Date Milestones
H1 2021
IND Filing and Study Start
H2 2021
NHV SAD/MAD data
H2 2021
Patient cohort in MAD
• Randomized, pbo-controlled, dose
escalation study
• SAD and MAD (14 daily doses)
• Up to 100 adult healthy volunteers
Primary endpoint: Safety
• Secondary endpoints: PK and PD (POB)
• IRAK4 levels in blood and skin
• Levels of pro-inflammatory
cytokines
• Ex-vivo stimulation of PBMC
• Plasma levels of hsCRP
• Small patient cohort of top MAD dose
to confirm PKPD
POB
Phase 2
Target Date Milestones
2022
Clinical POC
• Randomized, pbo-controlled,
study in pts in indications such
as HS, AD, RA
POCView entire presentation