Kymera Investor Presentation Deck
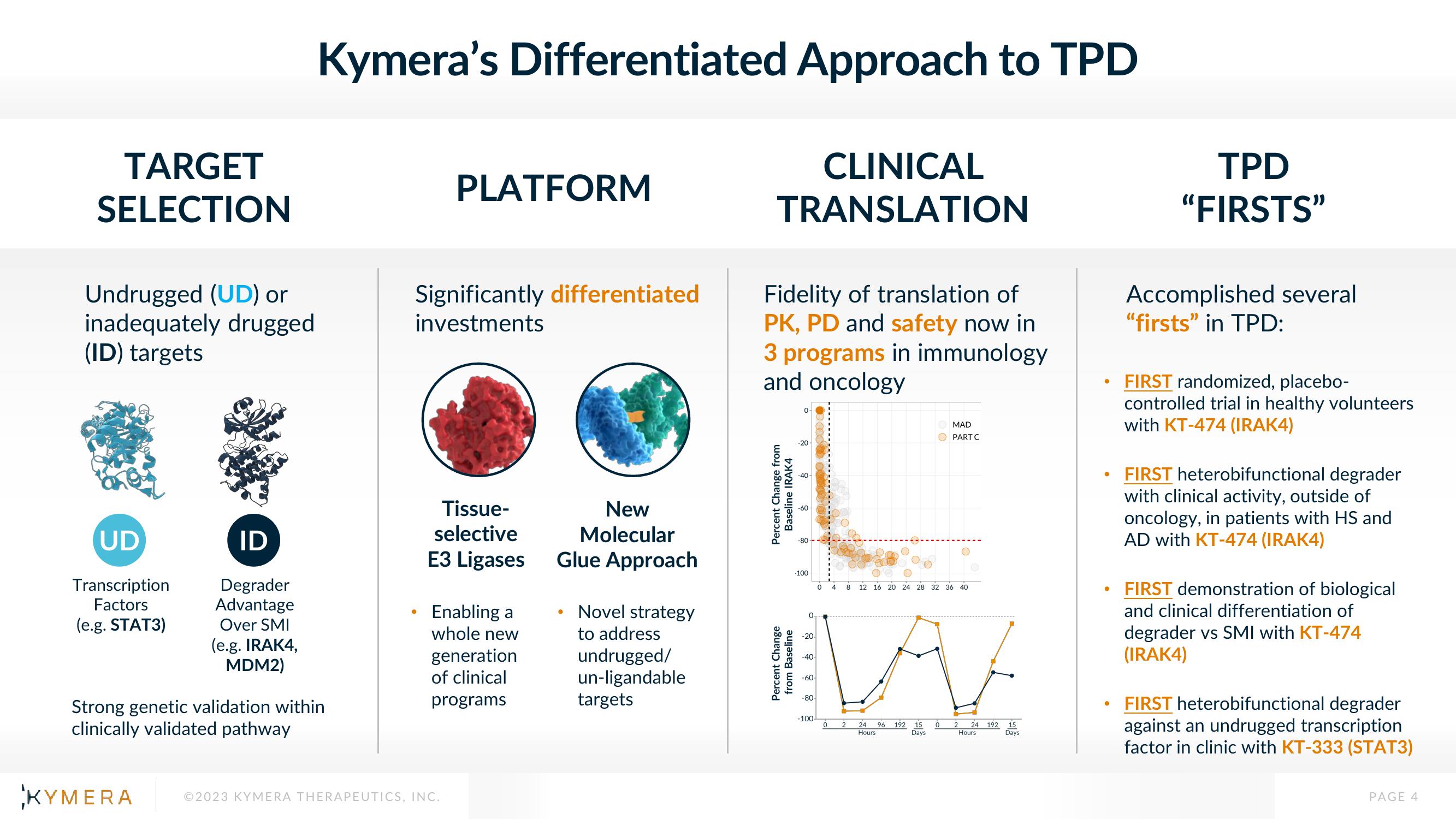
TARGET
SELECTION
Undrugged (UD) or
inadequately drugged
(ID) targets
UD
Transcription
Factors
(e.g. STAT3)
ID
Degrader
Advantage
Over SMI
(e.g. IRAK4,
MDM2)
KYMERA
Kymera's Differentiated Approach to TPD
CLINICAL
TRANSLATION
Strong genetic validation within
clinically validated pathway
PLATFORM
Significantly differentiated
investments
Tissue-
selective
E3 Ligases
Enabling a
whole new
generation
of clinical
programs
©2023 KYMERA THERAPEUTICS, INC.
New
Molecular
Glue Approach
Novel strategy
to address
undrugged/
un-ligandable
targets
Fidelity of translation of
PK, PD and safety now in
3 programs in immunology
and oncology
Percent Change from
Baseline IRAK4
Percent Change
from Baseline
-20
-100
0
-20-
-40-
-80
-100-
0 4
0
8
12 16 20 24 28 32 36 40
2 24 96 192 15
Hours
Days
MAD
PART C
0
2 24 192 15
Hours:
Days
●
TPD
"FIRSTS"
Accomplished several
"firsts" in TPD:
FIRST randomized, placebo-
controlled trial in healthy volunteers
with KT-474 (IRAK4)
FIRST heterobifunctional degrader
with clinical activity, outside of
oncology, in patients with HS and
AD with KT-474 (IRAK4)
FIRST demonstration of biological
and clinical differentiation of
degrader vs SMI with KT-474
(IRAK4)
FIRST heterobifunctional degrader
against an undrugged transcription
factor in clinic with KT-333 (STAT3)
PAGE 4View entire presentation