Ocuphire Pharma Investor Day Presentation Deck
JJ
는
112
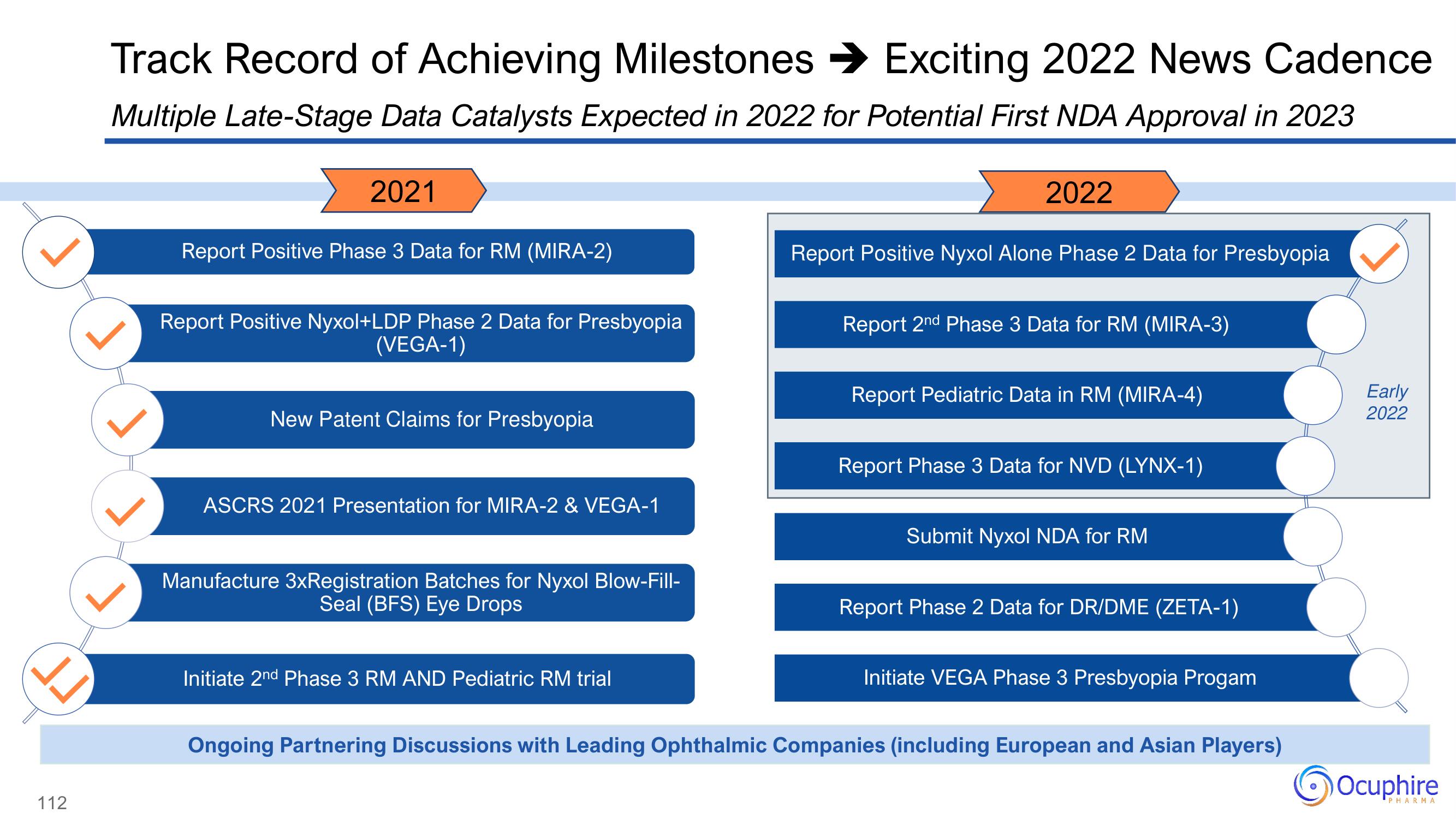
Track Record of Achieving Milestones ➜ Exciting 2022 News Cadence
→
Multiple Late-Stage Data Catalysts Expected in 2022 for Potential First NDA Approval in 2023
2021
Report Positive Phase 3 Data for RM (MIRA-2)
Report Positive Nyxol+LDP Phase 2 Data for Presbyopia
(VEGA-1)
New Patent Claims for Presbyopia
ASCRS 2021 Presentation for MIRA-2 & VEGA-1
Manufacture 3xRegistration Batches for Nyxol Blow-Fill-
Seal (BFS) Eye Drops
Initiate 2nd Phase 3 RM AND Pediatric RM trial
2022
Report Positive Nyxol Alone Phase 2 Data for Presbyopia
Report 2nd Phase 3 Data for RM (MIRA-3)
Report Pediatric Data in RM (MIRA-4)
Report Phase 3 Data for NVD (LYNX-1)
Submit Nyxol NDA for RM
Report Phase 2 Data for DR/DME (ZETA-1)
Initiate VEGA Phase 3 Presbyopia Progam
Ongoing Partnering Discussions with Leading Ophthalmic Companies (including European and Asian Players)
Early
2022
Ocuphire
PHARMAView entire presentation