Bausch+Lomb Results Presentation Deck
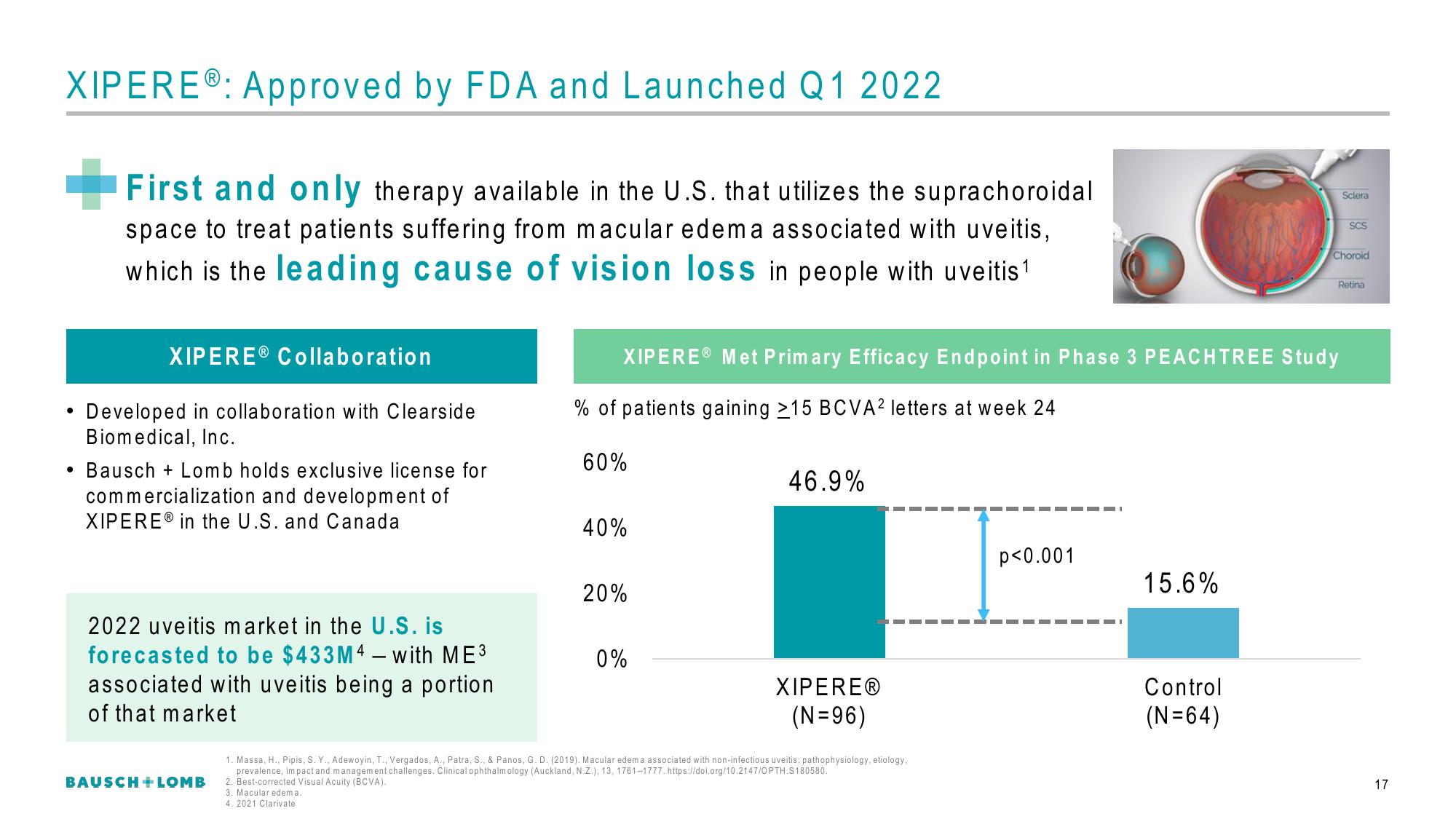
XIPEREⓇ: Approved by FDA and Launched Q1 2022
First and only therapy available in the U.S. that utilizes the suprachoroidal
space to treat patients suffering from macular edema associated with uveitis,
which is the leading cause of vision loss in people with uveitis ¹
XIPERE® Collaboration
Developed in collaboration with Clearside
Biomedical, Inc.
Bausch+Lomb holds exclusive license for
commercialization and development of
XIPERE® in the U.S. and Canada
2022 uveitis market in the U.S. is
forecasted to be $433M4 - with ME³
associated with uveitis being a portion
of that market
BAUSCH + LOMB
40%
XIPERE® Met Primary Efficacy Endpoint in Phase 3 PEACHTREE Study
% of patients gaining 215 BCVA2 letters at week 24
60%
20%
0%
46.9%
XIPEREⓇ
(N=96)
1. Massa, H., Pipis, S. Y., Adewoyin, T., Vergados, A., Patra, S., & Panos, G. D. (2019). Macular edema associated with non-infectious uveitis: pathophysiology, etiology,
prevalence, impact and management challenges. Clinical ophthalmology (Auckland, N.Z.), 13, 1761-1777. https://doi.org/10.2147/OPTH.S180580.
2. Best-corrected Visual Acuity (BCVA).
3. Macular edema.
4. 2021 Clarivate
p<0.001
T
15.6%
Control
(N=64)
Sclera
SCS
Choroid
Retina
17View entire presentation