AstraZeneca Investor Day Presentation Deck
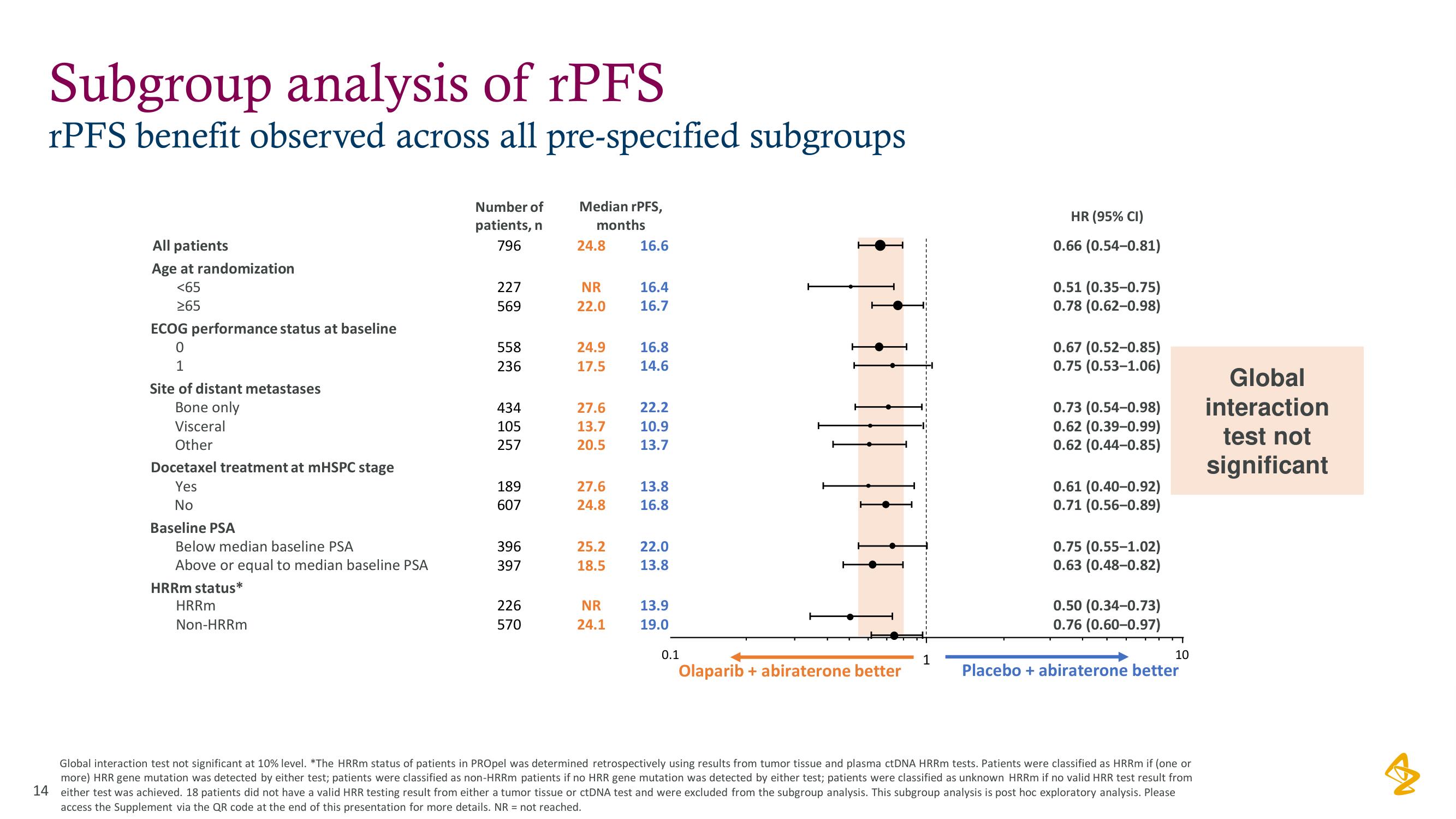
Subgroup analysis of rPFS
rPFS benefit observed across all pre-specified subgroups
All patients
Age at randomization
<65
265
ECOG performance status at baseline
1
Site of distant metastases
Bone only
Visceral
Other
Docetaxel treatment at mHSPC stage
Yes
No
Baseline PSA
Below median baseline PSA
Above or equal to median baseline PSA
HRRm status*
HRRm
Non-HRRm
Number of
patients, n
796
227
569
558
236
434
105
257
189
607
396
397
226
570
Median rPFS,
months
24.8
NR
22.0
24.9
17.5
27.6
13.7
20.5
27.6
24.8
25.2
18.5
NR
24.1
16.6
16.4
16.7
16.8
14.6
22.2
10.9
13.7
13.8
16.8
22.0
13.8
13.9
19.0
0.1
Olaparib + abiraterone better
1
HR (95% CI)
0.66 (0.54-0.81)
0.51 (0.35-0.75)
0.78 (0.62-0.98)
0.67 (0.52-0.85)
0.75 (0.53-1.06)
0.73 (0.54-0.98)
0.62 (0.39-0.99)
0.62 (0.44-0.85)
0.61 (0.40-0.92)
0.71 (0.56-0.89)
0.75 (0.55-1.02)
0.63 (0.48-0.82)
0.50 (0.34-0.73)
0.76 (0.60-0.97)
10
Placebo + abiraterone better
Global interaction test not significant at 10% level. *The HRRm status of patients in PROpel was determined retrospectively using results from tumor tissue and plasma ctDNA HRRm tests. Patients were classified as HRRm if (one or
more) HRR gene mutation was detected by either test; patients were classified as non-HRRm patients if no HRR gene mutation was detected by either test; patients were classified as unknown HRRm if no valid HRR test result from
14 either test was achieved. 18 patients did not have a valid HRR testing result from either a tumor tissue or ctDNA test and were excluded from the subgroup analysis. This subgroup analysis is post hoc exploratory analysis. Please
access the Supplement via the QR code at the end of this presentation for more details. NR = not reached.
Global
interaction
test not
significant
BView entire presentation