Ocuphire Pharma Investor Day Presentation Deck
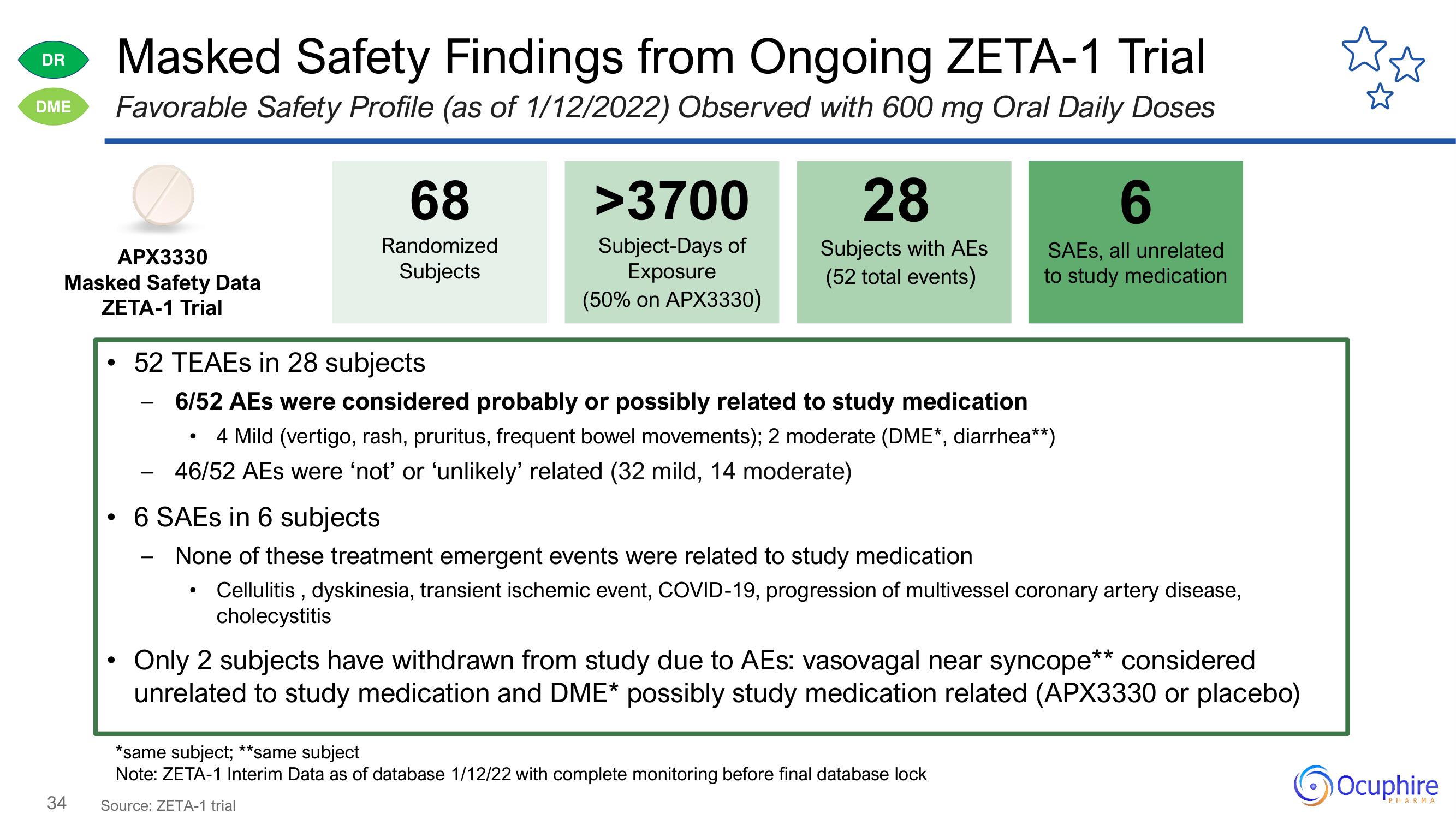
Masked Safety Findings from Ongoing ZETA-1 Trial
DME Favorable Safety Profile (as of 1/12/2022) Observed with 600 mg Oral Daily Doses
DR
APX3330
Masked Safety Data
ZETA-1 Trial
34
●
68
Randomized
Subjects
>3700
Subject-Days of
Exposure
(50% on APX3330)
●
28
Subjects with AEs
(52 total events)
• 52 TEAES in 28 subjects
6/52 AEs were considered probably or possibly related to study medication
●
• 4 Mild (vertigo, rash, pruritus, frequent bowel movements); 2 moderate (DME*, diarrhea**)
46/52 AEs were 'not' or 'unlikely' related (32 mild, 14 moderate)
6
SAES, all unrelated
to study medication
6 SAEs in 6 subjects
None of these treatment emergent events were related to study medication
Cellulitis, dyskinesia, transient ischemic event, COVID-19, progression of multivessel coronary artery disease,
cholecystitis
Only 2 subjects have withdrawn from study due to AEs: vasovagal near syncope** considered
unrelated to study medication and DME* possibly study medication related (APX3330 or placebo)
*same subject; **same subject
Note: ZETA-1 Interim Data as of database 1/12/22 with complete monitoring before final database lock
Source: ZETA-1 trial
Ocuphire
PHARMAView entire presentation