Kymera Investor Presentation Deck
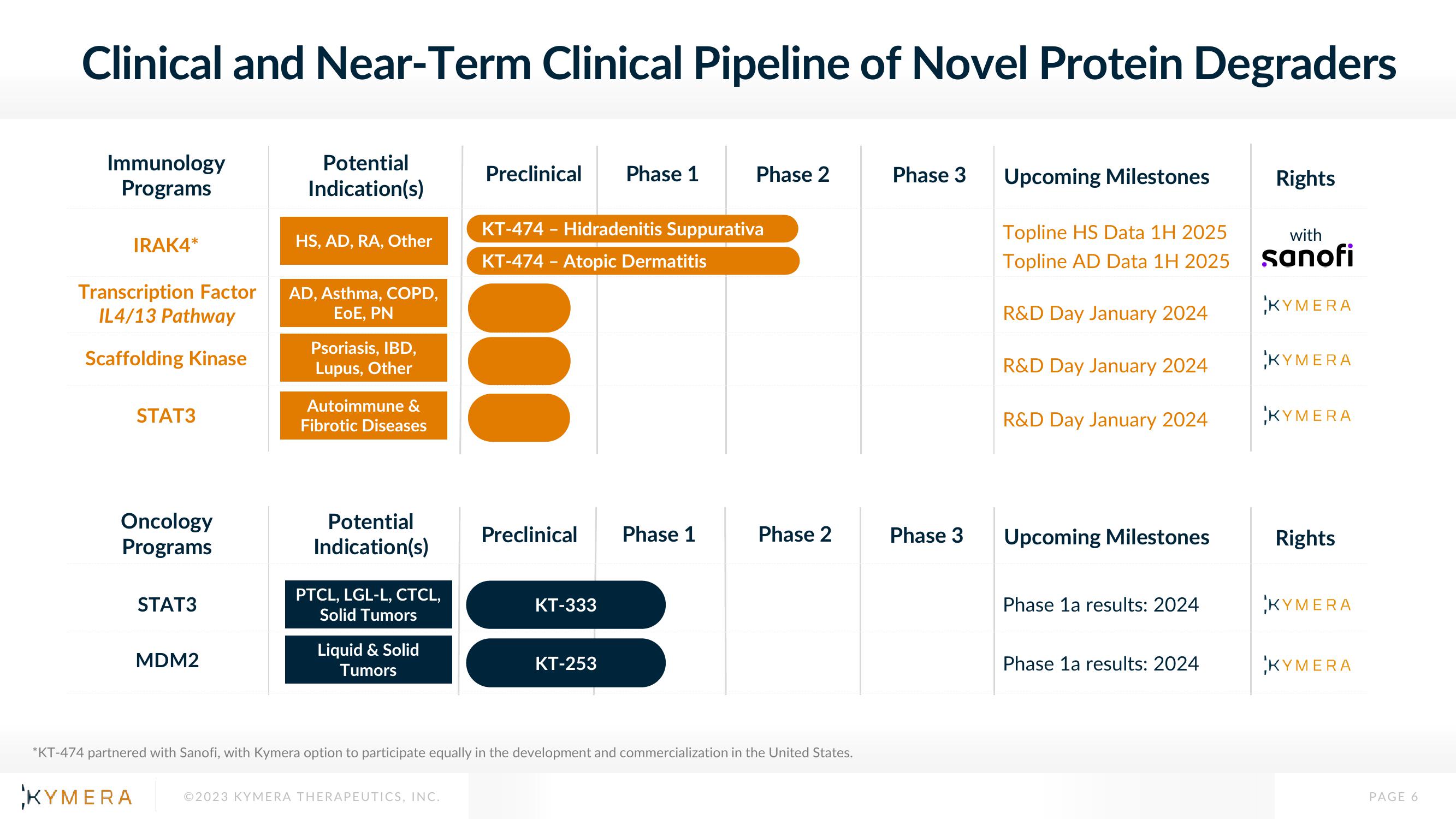
Clinical and Near-Term Clinical Pipeline of Novel Protein Degraders
Immunology
Programs
IRAK4*
Transcription Factor
IL4/13 Pathway
Scaffolding Kinase
STAT3
Oncology
Programs
STAT3
MDM2
Potential
Indication(s)
HS, AD, RA, Other
AD, Asthma, COPD,
EoE, PN
Psoriasis, IBD,
Lupus, Other
Autoimmune &
Fibrotic Diseases
Potential
Indication(s)
PTCL, LGL-L, CTCL,
Solid Tumors
Liquid & Solid
Tumors
Preclinical Phase 1
KYMERA Ⓒ2023 KYMERA THERAPEUTICS, INC.
KT-474 - Hidradenitis Suppurativa
KT-474 - Atopic Dermatitis
00
Preclinical
KT-333
KT-253
Phase 2
Phase 1
Phase 2
*KT-474 partnered with Sanofi, with Kymera option to participate equally in the development and commercialization in the United States.
Phase 3
Phase 3
Upcoming Milestones
Topline HS Data 1H 2025
Topline AD Data 1H 2025
R&D Day January 2024
R&D Day January 2024
R&D Day January 2024
Upcoming Milestones
Phase 1a results: 2024
Phase 1a results: 2024
Rights
with
sanofi
KYMERA
KYMERA
KYMERA
Rights
KYMERA
KYMERA
PAGE 6View entire presentation