Pershing Square Activist Presentation Deck
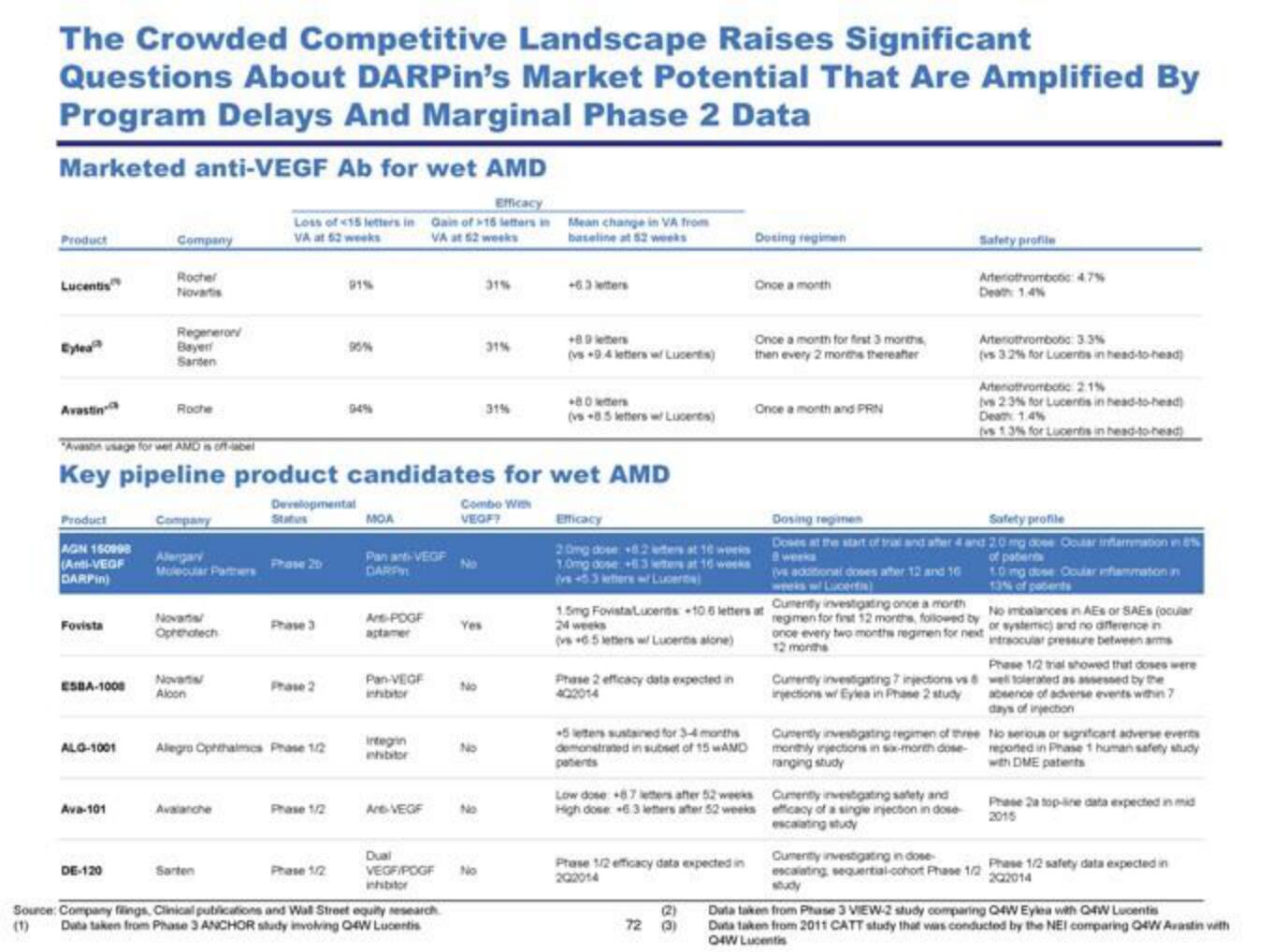
The Crowded Competitive Landscape Raises Significant
Questions About DARPin's Market Potential That Are Amplified By
Program Delays And Marginal Phase 2 Data
Marketed anti-VEGF Ab for wet AMD
Efficacy
Loss of 15 letters in Gain of 16 letters in
VA at 52 weeks
VA at 52 weeks
Product
Lucentis
Eylea
Avastin
Product
AGN 160998
(Anti-VEGF
DARPIN)
Fovista
ESBA-1008
ALG-1001
Ava-101
Company
Rocher
Novartis
DE-120
Regenerov
Bayer
Santen
Roche
Company
Alergary
Molecular Paters
"Avason usage for wet AMD is off-label
Key pipeline product candidates for wet AMD
Developeriavitat
Combo With
VEGF?
Novartal
Oprenotech
Alcon
Avalanche
Phase 20
Sarten
Phase 3
Allegro Ophthalmics Phase 1/2
Phase 2
Phase 1/2
91%
Phase 1/2
90%
MOA
Panant-VEGF
DARP
Art-POGF
aptamer
Pan-VEGF
inhibitor
integrin
nhibitor
Are-VEGF
Dual
VEGF/POGF
inhibitor
Source: Company filings, Clinical publications and Wall Street equity research
(1) Duta taken from Phase 3 ANCHOR study involving O4W Lucentis
No
Yes
No
No
No
31%
No
31%
Mean change in VA from
baseline at 52 weeks
+6.3 letters
+80 letters
(vs 9.4 letters wf Lucents)
+80 letters
(vs+85 letters w Lucents)
Efficacy
20mg dose 82 ters at 10 works
10mg done +63 eters at 16 weeks
(vs+53 ters / Lucer
24 weeks
(vs 65 letters w/ Lucents alone)
Phase 2 efficacy data expected in
402014
5 letters sustained for 3-4 months
demonstrated in subset of 15 wAMD
Dosing regimen
Prase 1/2 efficacy data expected in
202014
Once a month
(2)
72 (3)
Once a month for first 3 months
then every 2 months thereafter
Once a month and PRN
Cumenty investigating once a month
No imbalances in AES or SAEs (ocular
1.5mg FovistaLucents +10 6 letters at regimen for first 12 months, followed by, or systemic) and no difference in
once every two months regimen for next intraocular pressure between arms
12 months
Low dose +87 letters after 52 weeks
High dose 6.3 letters after 52 weeks
Safety profile
Arterothromboc: 47%
Death 1.4%
Currently investigating injections vs
injections w/ Eylea in Phase 2 study
Arterothrombotic: 3.3%
(vs 3.2% for Lucents in head-to-head)
Dosing regimen
Safety profile
8 weeks
Doses at the start of tral and after 4 and 20 mg dose Ocular inflammation in 8%
of patients
10 mg dose Ocular inflammation in
13% of patents
vs additional doses after 12 and 16
Artenothrombotic 2.1%
vs 23% for Lucentis in head-to-head)
Death 1.4%
(vs 1.3% for Lucents in head-to-head)
Cumenty investigating safety and
efficacy of a single injection in dose
escalating study
Cumenty investigating regimen of three
monthly injections in six-morth dose
ranging study
Phase 1/2 trial showed that doses were
well tolerated as assessed by the
absence of adverse events within 7
days of injection
No serious or significant adverse events
reported in Phase 1 human safety study
with DME patients
Phase 2a top-line data expected in mid
2015
Cumenty investigating in dose
escalating sequential-cohort Phase 1/2 Phase 1/2 safety data expected in
202014
Data taken from Phase 3 VIEW-2 study comparing Q4W Eylea with Q4W Lucentis
Data taken from 2011 CATT study that was conducted by the NEI comparing Q4W Awastin with
04W LucentisView entire presentation