Ocuphire Pharma Investor Presentation Deck
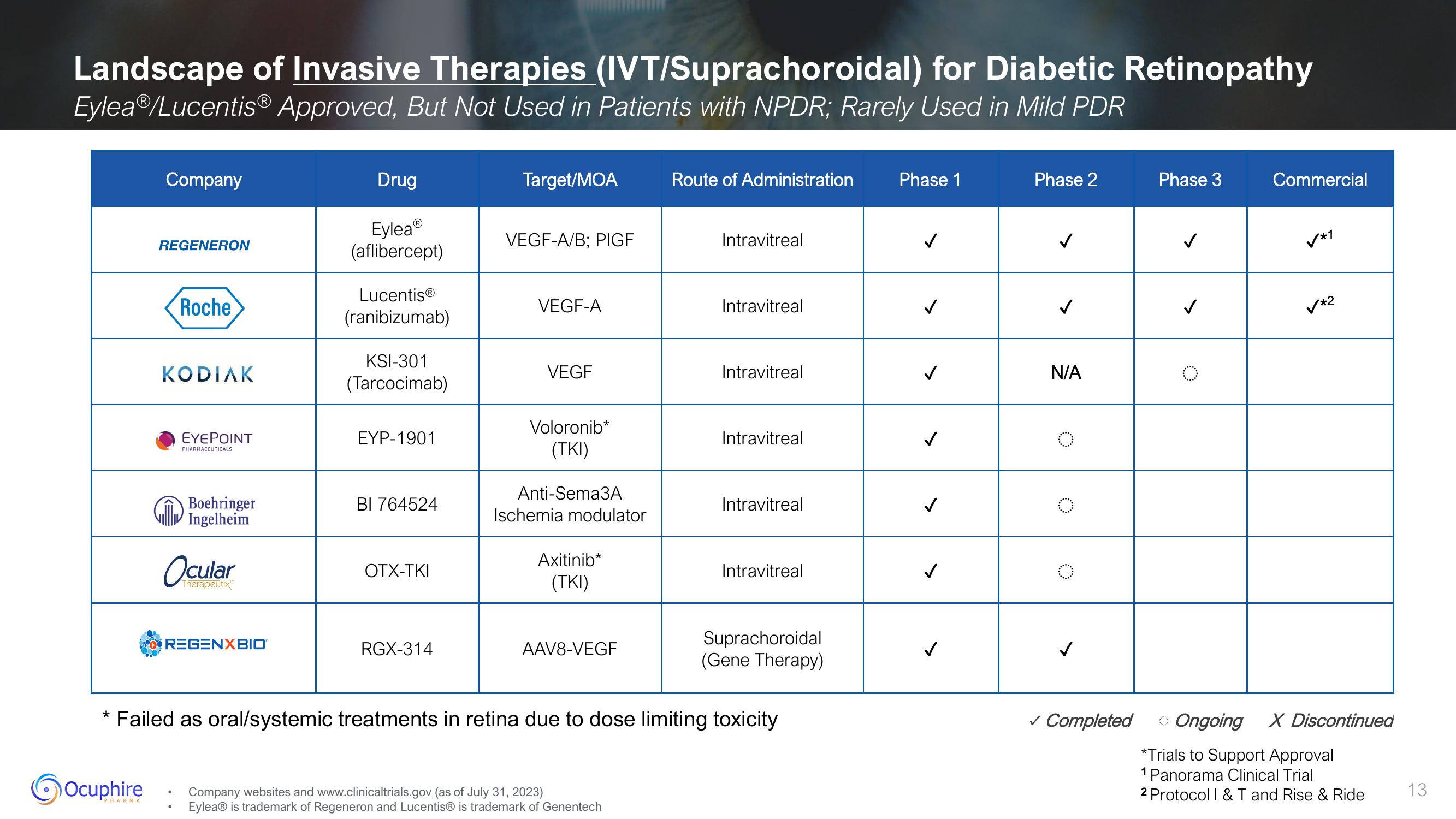
Landscape of Invasive Therapies (IVT/Suprachoroidal) for Diabetic Retinopathy
EyleaⓇ/Lucentis® Approved, But Not Used in Patients with NPDR; Rarely Used in Mild PDR
Company
REGENERON
Roche
KODIAK
EYEPOINT
PHARMACEUTICALS
Boehringer
Ingelheim
Therapeutix
REGENXBIO®
Drug
Eylea
(aflibercept)
Lucentis®
(ranibizumab)
KSI-301
(Tarcocimab)
EYP-1901
BI 764524
OTX-TKI
RGX-314
Target/MOA
VEGF-A/B; PIGF
VEGF-A
VEGF
Voloronib*
(TKI)
Anti-Sema3A
Ischemia modulator
Axitinib*
(TKI)
AAV8-VEGF
Ocuphire Company websites and www.clinical trials.gov (as of July 31, 2023)
Route of Administration
Eylea® is trademark of Regeneron and Lucentis® is trademark of Genentech
Intravitreal
Intravitreal
Intravitreal
Intravitreal
Intravitreal
Intravitreal
* Failed as oral/systemic treatments in retina due to dose limiting toxicity
Suprachoroidal
(Gene Therapy)
Phase 1
✓
✓
Phase 2
✓
N/A
Completed
Phase 3
Commercial
✓*1
✓*2
Ongoing X Discontinued
*Trials to Support Approval
1 Panorama Clinical Trial
2 Protocol I & T and Rise & Ride
13View entire presentation