BenevolentAI Results Presentation Deck
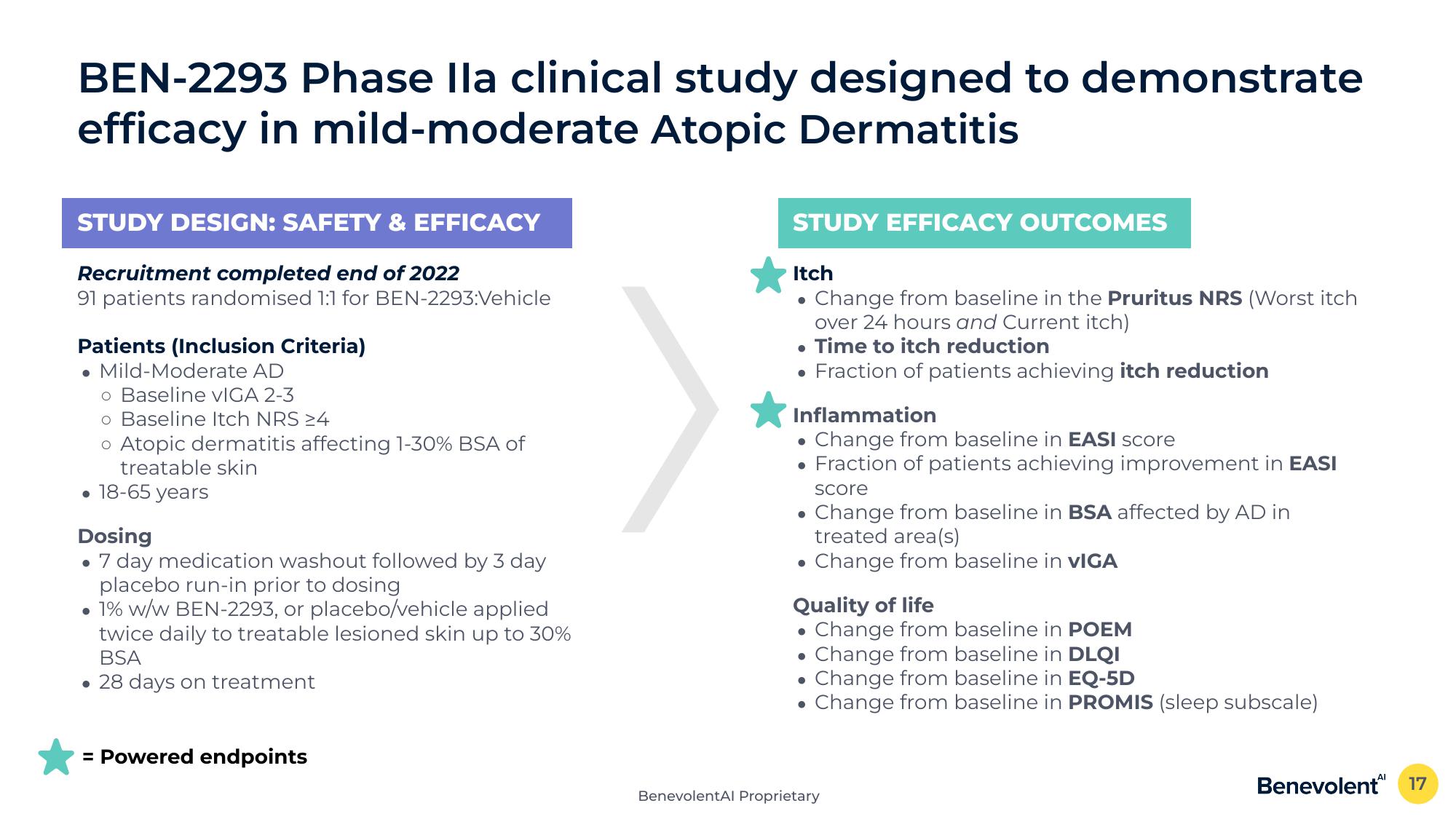
BEN-2293 Phase lla clinical study designed to demonstrate
efficacy in mild-moderate Atopic Dermatitis
STUDY DESIGN: SAFETY & EFFICACY
Recruitment completed end of 2022
91 patients randomised 1:1 for BEN-2293:Vehicle
Patients (Inclusion Criteria)
• Mild-Moderate AD
o Baseline VIGA 2-3
o Baseline Itch NRS 24
o Atopic dermatitis affecting 1-30% BSA of
treatable skin
• 18-65 years
Dosing
7 day medication washout followed by 3 day
placebo run-in prior to dosing
• 1% w/w BEN-2293, or placebo/vehicle applied
twice daily to treatable lesioned skin up to 30%
BSA
• 28 days on treatment
= Powered endpoints
STUDY EFFICACY OUTCOMES
Itch
• Change from baseline in the Pruritus NRS (Worst itch
over 24 hours and Current itch)
Time to itch reduction
●
• Fraction of patients achieving itch reduction
Inflammation
Change from baseline in EASI score
Fraction of patients achieving improvement in EASI
●
●
score
Change from baseline in BSA affected by AD in
treated area(s)
• Change from baseline in VIGA
Quality of life
Change from baseline in POEM
. Change from baseline in DLQI
• Change from baseline in EQ-5D
Change from baseline in PROMIS (sleep subscale)
●
BenevolentAl Proprietary
Benevolent 17View entire presentation