Vaxcyte Corporate Presentation
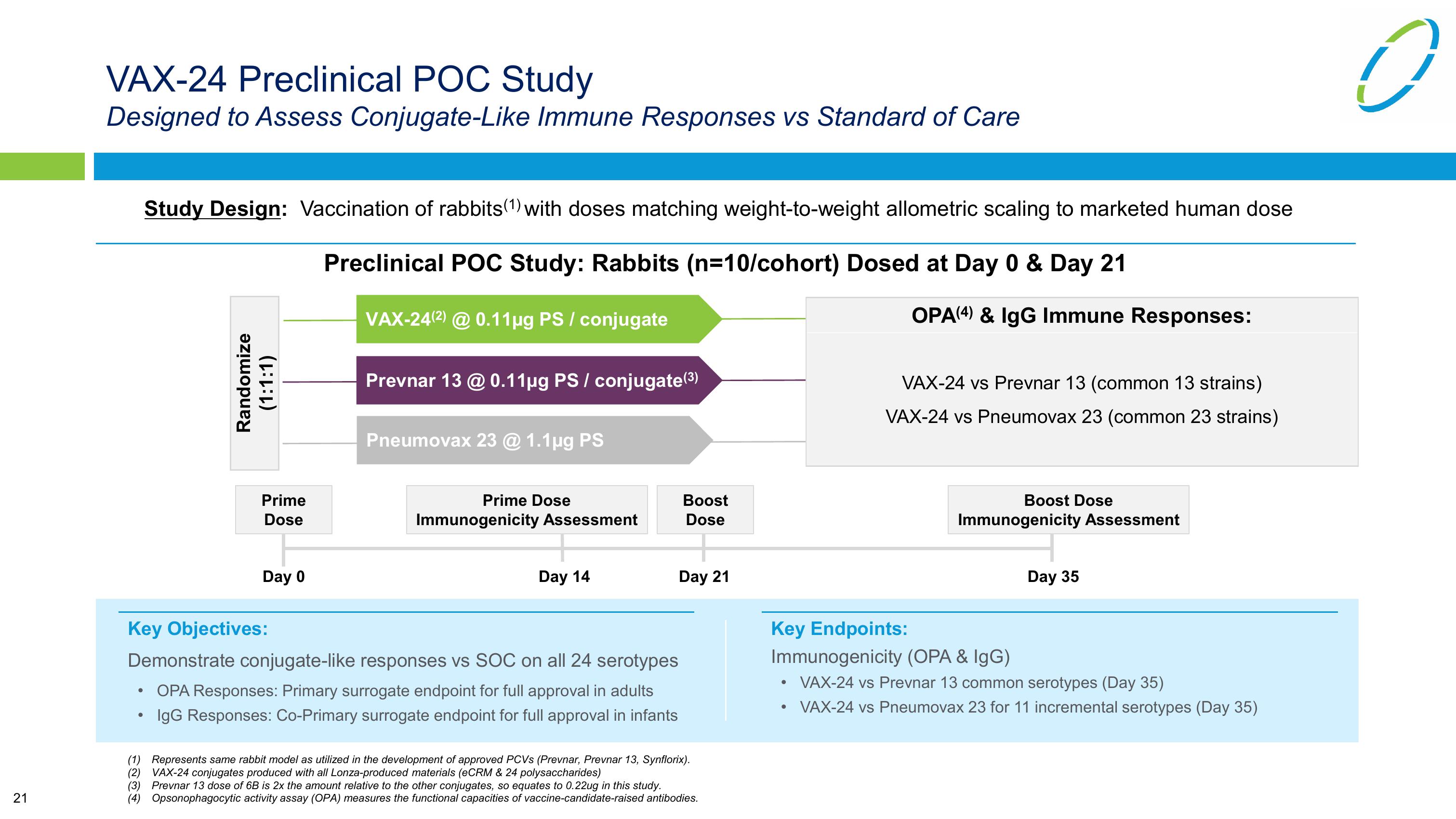
VAX-24 Preclinical POC Study
Designed to Assess Conjugate-Like Immune Responses vs Standard of Care
21
21
Study Design: Vaccination of rabbits (1) with doses matching weight-to-weight allometric scaling to marketed human dose
Preclinical POC Study: Rabbits (n=10/cohort) Dosed at Day 0 & Day 21
Randomize
(1:1:1)
VAX-24(2) @ 0.11μg PS / conjugate
Prevnar 13 @ 0.11µg PS / conjugate(3)
Pneumovax 23 @ 1.1μg PS
OPA (4) & IgG Immune Responses:
VAX-24 vs Prevnar 13 (common 13 strains)
VAX-24 vs Pneumovax 23 (common 23 strains)
Prime
Prime Dose
Dose
Immunogenicity Assessment
Boost
Dose
Day 0
Day 14
Day 21
Key Endpoints:
Boost Dose
Immunogenicity Assessment
Day 35
Key Objectives:
Demonstrate conjugate-like responses vs SOC on all 24 serotypes
•
OPA Responses: Primary surrogate endpoint for full approval in adults
IgG Responses: Co-Primary surrogate endpoint for full approval in infants
Immunogenicity (OPA & IgG)
VAX-24 vs Prevnar 13 common serotypes (Day 35)
VAX-24 vs Pneumovax 23 for 11 incremental serotypes (Day 35)
(1) Represents same rabbit model as utilized in the development of approved PCVs (Prevnar, Prevnar 13, Synflorix).
(2) VAX-24 conjugates produced with all Lonza-produced materials (eCRM & 24 polysaccharides)
(3) Prevnar 13 dose of 6B is 2x the amount relative to the other conjugates, so equates to 0.22ug in this study.
(4) Opsonophagocytic activity assay (OPA) measures the functional capacities of vaccine-candidate-raised antibodies.
оView entire presentation