Vaxcyte Corporate Presentation
26
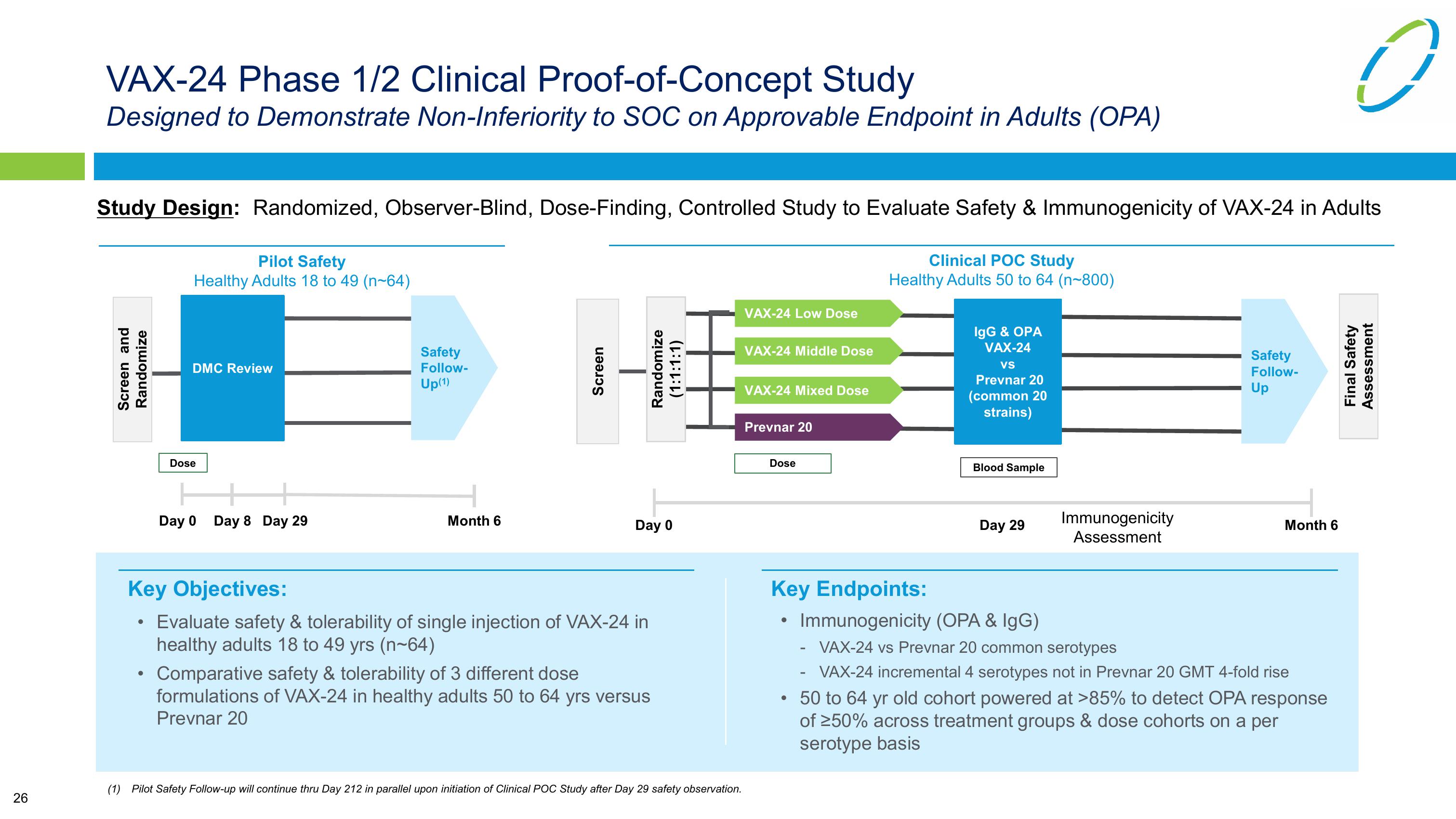
VAX-24 Phase 1/2 Clinical Proof-of-Concept Study
Designed to Demonstrate Non-Inferiority to SOC on Approvable Endpoint in Adults (OPA)
о
Study Design: Randomized, Observer-Blind, Dose-Finding, Controlled Study to Evaluate Safety & Immunogenicity of VAX-24 in Adults
Screen and
Randomize
Pilot Safety
Healthy Adults 18 to 49 (n-64)
DMC Review
Safety
Follow-
Up(1)
Screen
Randomize
(1:1:1:1)
VAX-24 Low Dose
VAX-24 Middle Dose
VAX-24 Mixed Dose
Clinical POC Study
Healthy Adults 50 to 64 (n-800)
IgG & OPA
VAX-24
VS
Prevnar 20
(common 20
strains)
Safety
Follow-
Up
Final Safety
Assessment
Dose
Day 0 Day 8 Day 29
Month 6
Day 0
Key Objectives:
• Evaluate safety & tolerability of single injection of VAX-24 in
healthy adults 18 to 49 yrs (n~64)
Comparative safety & tolerability of 3 different dose
.
formulations of VAX-24 in healthy adults 50 to 64 yrs versus
Prevnar 20
(1) Pilot Safety Follow-up will continue thru Day 212 in parallel upon initiation of Clinical POC Study after Day 29 safety observation.
Prevnar 20
Dose
Key Endpoints:
•
Blood Sample
Day 29
Immunogenicity
Assessment
Month 6
Immunogenicity (OPA & IgG)
- VAX-24 vs Prevnar 20 common serotypes
-
VAX-24 incremental 4 serotypes not in Prevnar 20 GMT 4-fold rise
50 to 64 yr old cohort powered at >85% to detect OPA response
of ≥50% across treatment groups & dose cohorts on a per
serotype basisView entire presentation