AstraZeneca Results Presentation Deck
Percentage mean change in UACR
from baseline to week 12
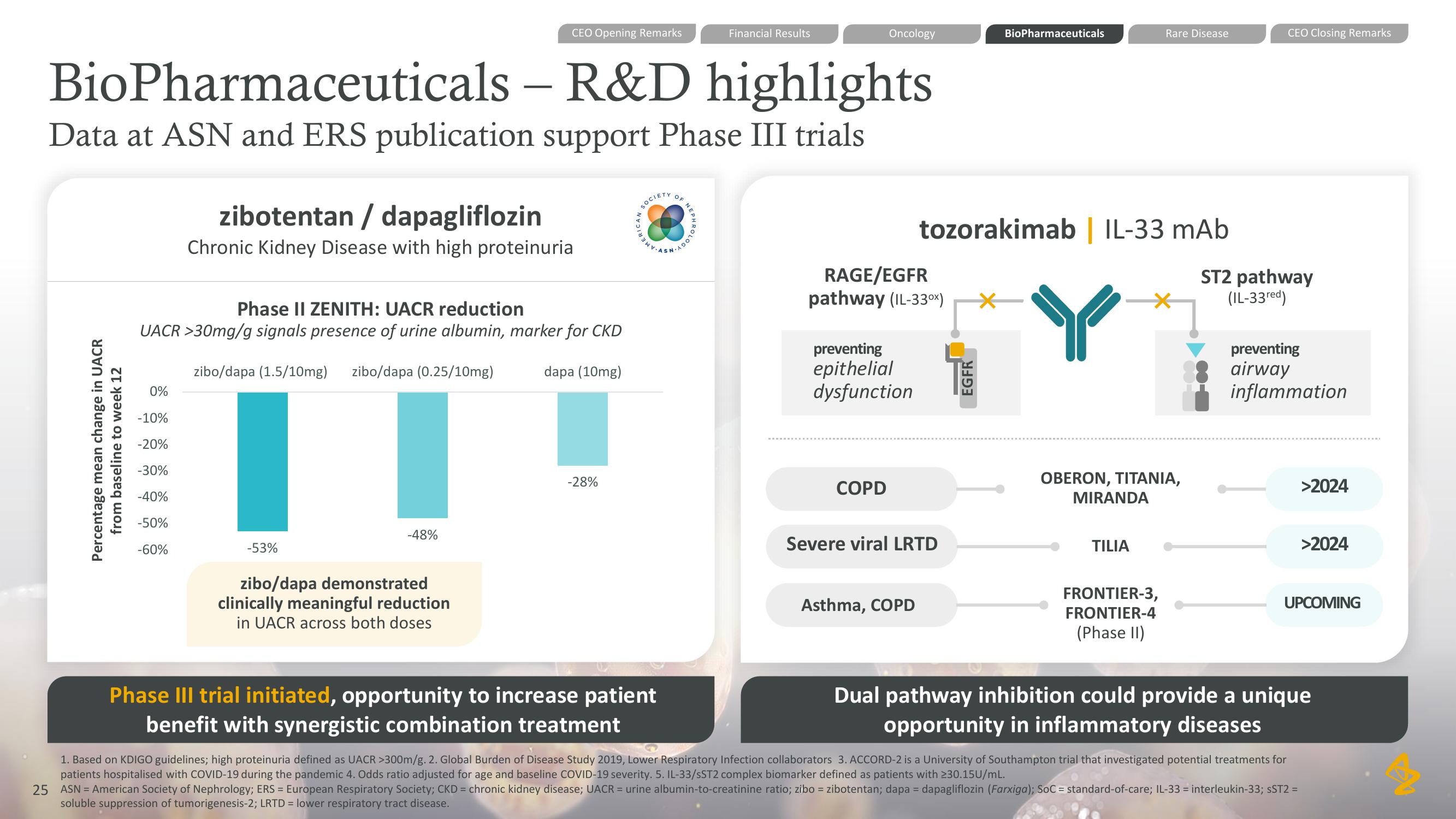
BioPharmaceuticals - R&D highlights
Data at ASN and ERS publication support Phase III trials
0%
-10%
-20%
-30%
-40%
-50%
-60%
zibotentan / dapagliflozin
Chronic Kidney Disease with high proteinuria
Phase II ZENITH: UACR reduction
UACR >30mg/g signals presence of urine albumin, marker for CKD
zibo/dapa (1.5/10mg) zibo/dapa (0.25/10mg)
CEO Opening Remarks
-53%
-48%
zibo/dapa demonstrated
clinically meaningful reduction
in UACR across both doses
dapa (10mg)
-28%
SOCIETY
Phase III trial initiated, opportunity to increase patient
benefit with synergistic combination treatment
OF
LOGI
Financial Results
១០
Oncology
RAGE/EGFR
pathway (IL-33⁰x)
preventing
epithelial
dysfunction
COPD
Severe viral LRTD
Asthma, COPD
tozorakimab | IL-33 mAb
BioPharmaceuticals
EGFR
Y
OBERON, TITANIA,
MIRANDA
Rare Disease
TILIA
FRONTIER-3,
FRONTIER-4
(Phase II)
CEO Closing Remarks
ST2 pathway
(IL-33red)
preventing
airway
inflammation
>2024
>2024
UPCOMING
1. Based on KDIGO guidelines; high proteinuria defined as UACR >300m/g. 2. Global Burden of Disease Study 2019, Lower Respiratory Infection collaborators 3. ACCORD-2 is a University of Southampton trial that investigated potential treatments for
patients hospitalised with COVID-19 during the pandemic 4. Odds ratio adjusted for age and baseline COVID-19 severity. 5. IL-33/SST2 complex biomarker defined as patients with 230.15U/mL.
25 ASN = American Society of Nephrology; ERS = European Respiratory Society; CKD = chronic kidney disease; UACR = urine albumin-to-creatinine ratio; zibo = zibotentan; dapa = dapagliflozin (Farxiga); SoC = standard-of-care; IL-33 = interleukin-33; SST2 =
soluble suppression of tumorigenesis-2; LRTD = lower respiratory tract disease.
Dual pathway inhibition could provide a unique
opportunity in inflammatory diseasesView entire presentation