AstraZeneca Results Presentation Deck
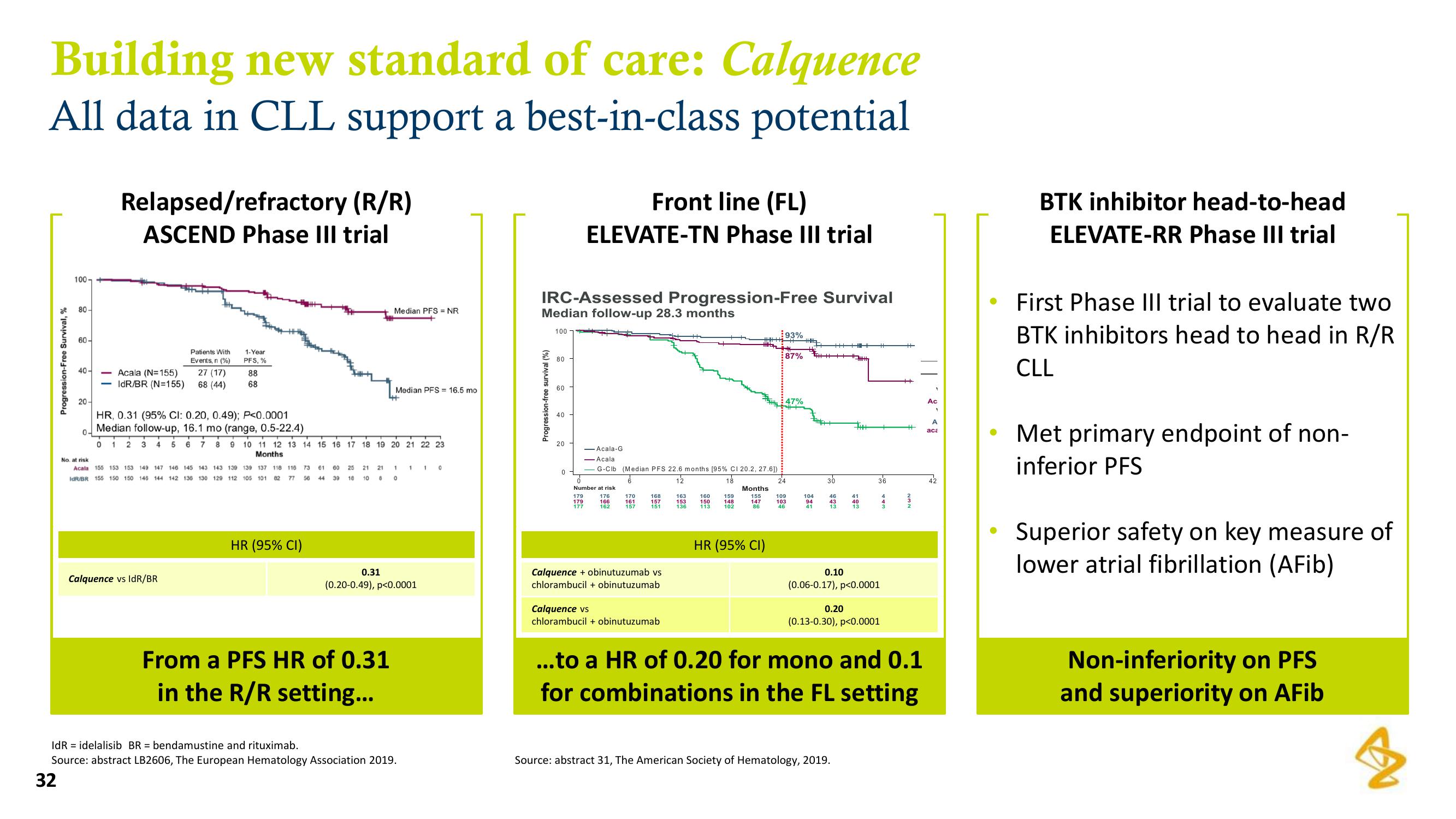
Building new standard of care: Calquence
All data in CLL support a best-in-class potential
Progression-Free Survival, %
100-
80-
60-
40-
20-
0-
Relapsed/refractory (R/R)
ASCEND Phase III trial
- Acala (N=155)
IdR/BR (N=155)
0
Patients With
Events, n (%)
27 (17)
68 (44)
Calquence vs IdR/BR
1-Year
PFS, %
88
68
HR, 0.31 (95% CI: 0.20, 0.49); P<0.0001
Median follow-up, 16.1 mo (range, 0.5-22.4)
2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23
Months
No. at risk
Acala 155 153 153 149 147 146 145 143 143 139 139 137 118 116 73
IdR/BR 155 150 150 145 144 142 136 130 129 112 105 101 82
56
HR (95% CI)
61
60
44 39
25
18
21
10
21
8
0.31
Median PFS = NR
Median PFS 16.5 mo
4+
From a PFS HR of 0.31
in the R/R setting...
1
0
(0.20-0.49), p<0.0001
1
IdRidelalisib BR = bendamustine and rituximab.
Source: abstract LB2606, The European Hematology Association 2019.
32
10
IRC-Assessed Progression-Free Survival
Median follow-up 28.3 months
Progression-free survival (%)
100+
80
60
40
Front line (FL)
ELEVATE-TN Phase III trial
20
0
Number at risk
179
177
Acala-G
Acala
G-Clb (Median PFS 22.6 months [95% CI 20.2, 27.6])
12
18
24
162
170
161
157
168
157
151
Calquence + obinutuzumab vs
chlorambucil + obinutuzumab
Calquence vs
chlorambucil + obinutuzumab
163
153
136
160
150
113
159
148
102
Months
155
147
86
93%
HR (95% CI)
87%
109
103
46
104
94
41
30
0.10
0.20
41 40 13
36
(0.06-0.17), p<0.0001
WAA
Source: abstract 31, The American Society of Hematology, 2019.
(0.13-0.30), p<0.0001
+
Nowhe
32
|| 28
...to a HR of 0.20 for mono and 0.1
for combinations in the FL setting
1
42
BTK inhibitor head-to-head
ELEVATE-RR Phase III trial
First Phase III trial to evaluate two
BTK inhibitors head to head in R/R
CLL
Met primary endpoint of non-
inferior PFS
Superior safety on key measure of
lower atrial fibrillation (AFib)
Non-inferiority on PFS
and superiority on AFib
ÂView entire presentation