Kymera Investor Presentation Deck
●
●
●
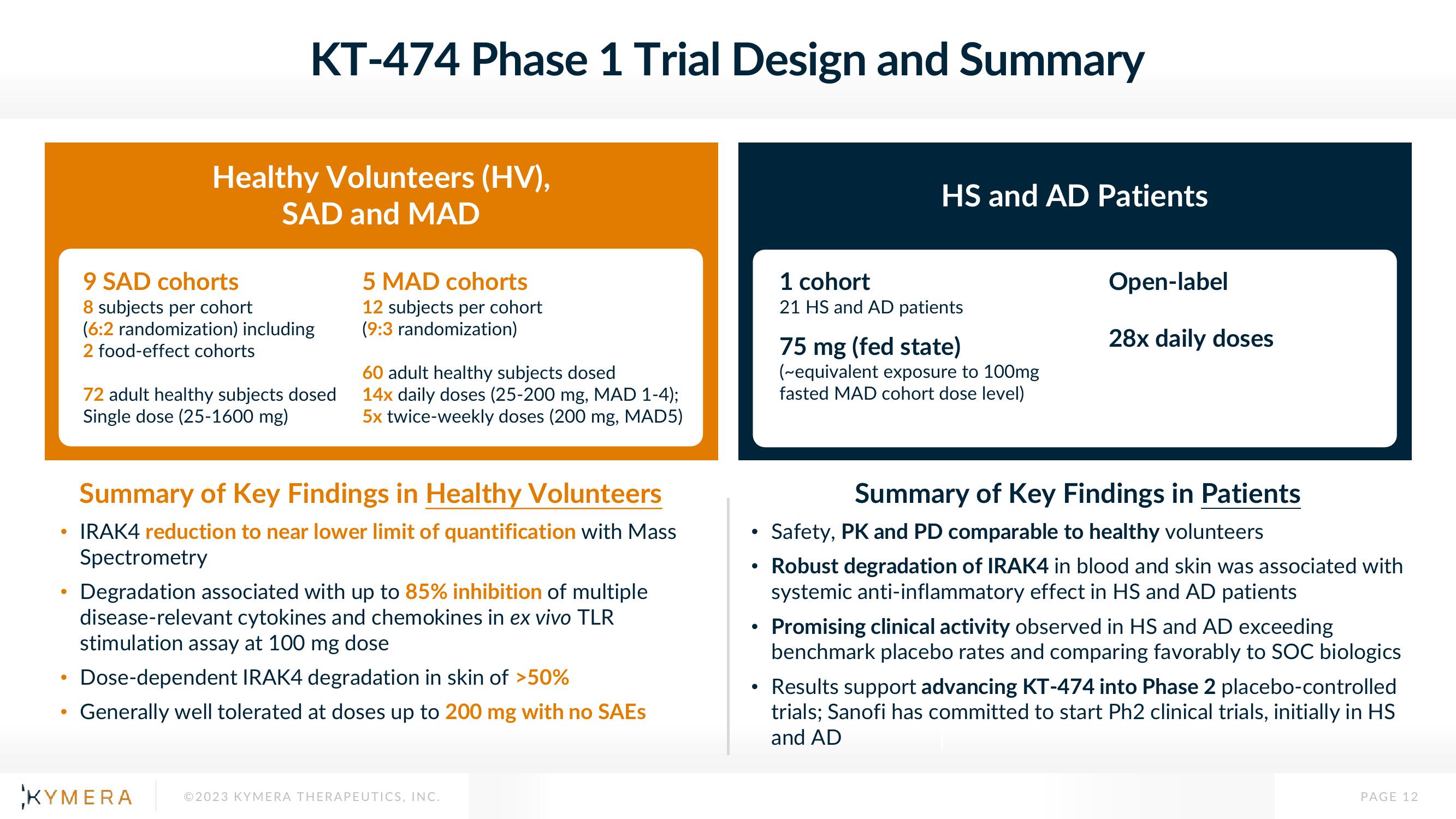
KT-474 Phase 1 Trial Design and Summary
Healthy Volunteers (HV),
SAD and MAD
9 SAD cohorts
8 subjects per cohort
(6:2 randomization) including
2 food-effect cohorts
72 adult healthy subjects dosed
Single dose (25-1600 mg)
5 MAD cohorts
12 subjects per cohort
(9:3 randomization)
60 adult healthy subjects dosed
14x daily doses (25-200 mg, MAD 1-4);
5x twice-weekly doses (200 mg, MAD5)
Summary of Key Findings in Healthy Volunteers
IRAK4 reduction to near lower limit of quantification with Mass
Spectrometry
Degradation associated with up to 85% inhibition of multiple
disease-relevant cytokines and chemokines in ex vivo TLR
stimulation assay at 100 mg dose
Dose-dependent IRAK4 degradation in skin of >50%
Generally well tolerated at doses up to 200 mg with no SAEs
KYMERA ©2023 KYMERA THERAPEUTICS, INC.
●
●
HS and AD Patients
1 cohort
21 HS and AD patients
75 mg (fed state)
(~equivalent exposure to 100mg
fasted MAD cohort dose level)
Open-label
28x daily doses
Summary of Key Findings in Patients
Safety, PK and PD comparable to healthy volunteers
Robust degradation of IRAK4 in blood and skin was associated with
systemic anti-inflammatory effect in HS and AD patients
Promising clinical activity observed in HS and AD exceeding
benchmark placebo rates and comparing favorably to SOC biologics
Results support advancing KT-474 into Phase 2 placebo-controlled
trials; Sanofi has committed to start Ph2 clinical trials, initially in HS
and AD
PAGE 12View entire presentation