Bausch+Lomb Investor Conference Presentation Deck
NOV03¹: PDUFA Date June 2023
Pivotal Phase 3 trial GOBI Data on NOV03 Now Published in Ophthalmology
Investigational first in class treatment for Dry Eye Disease (DED) associated
with Meibomian Gland Dysfunction (MGD)
Decrease from Baseline (%)
Consistent statistically significant efficacy, safety and tolerability have now
been demonstrated in two Phase 3 studies of NOV03¹ and one Phase 2 study
Statistically significant difference of sign and symptom was noted at day
15 and 57 in both Phase 3 studies
BAUSCH + LOMB
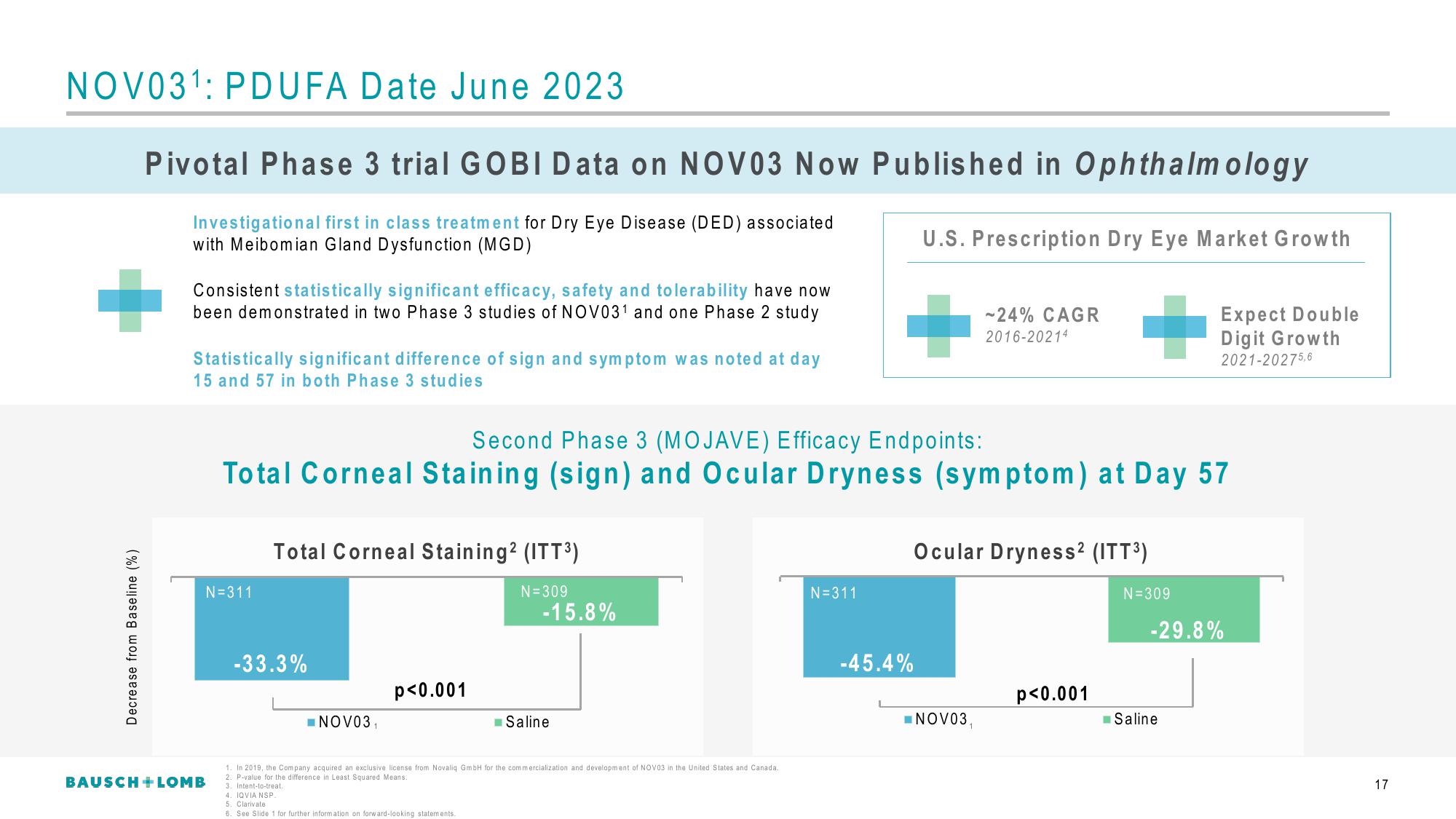
N=311
Total Corneal Staining² (ITT³)
N=309
-33.3%
Second Phase 3 (MOJAVE) Efficacy Endpoints:
Total Corneal Staining (sign) and Ocular Dryness (symptom) at Day 57
■NOV03₁
p<0.001
-15.8%
Saline
1. In 2019, the Company acquired an exclusive license from Novaliq GmbH for the commercialization and development of NOV03 in the United States and Canada.
2. P-value for the difference in Least Squared Means.
3. Intent-to-treat.
4. IQVIA NSP.
5. Clarivate.
6. See Slide 1 for further information on forward-looking statements.
N=311
U.S. Prescription Dry Eye Market Growth
+
-45.4%
-24% CAGR
2016-20214
Ocular Dryness² (ITT³)
■NOV03
p<0.001
N=309
Expect Double
Digit Growth
2021-20275,6
-29.8%
Saline
17View entire presentation