AstraZeneca Results Presentation Deck
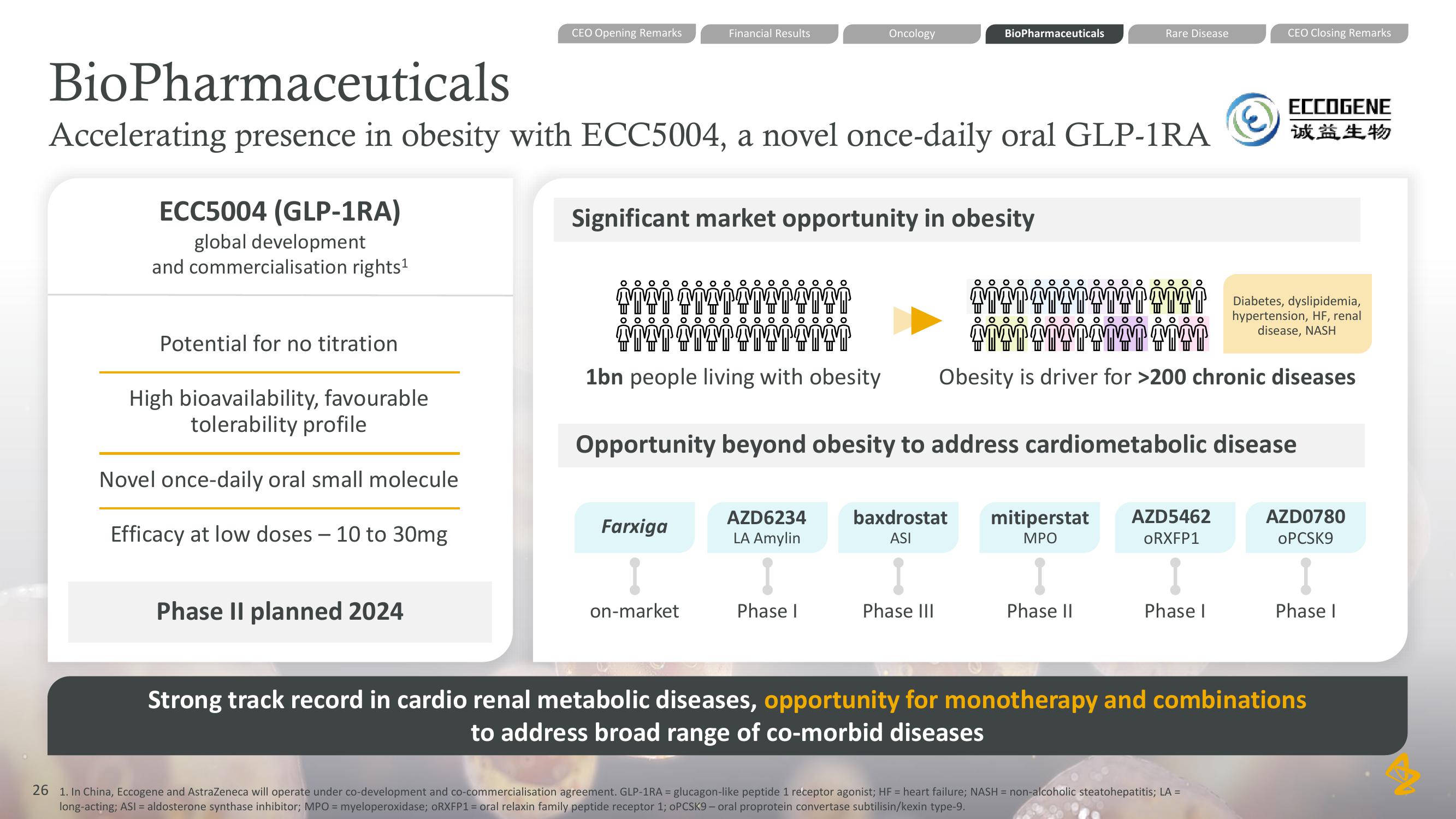
ECC5004 (GLP-1RA)
global development
and commercialisation rights ¹
Potential for no titration
High bioavailability, favourable
tolerability profile
Novel once-daily oral small molecule
Efficacy at low doses - 10 to 30mg
CEO Opening Remarks
Phase II planned 2024
BioPharmaceuticals
Accelerating presence in obesity with ECC5004, a novel once-daily oral GLP-1RA
Financial Results
Significant market opportunity in obesity
Farxiga
¡
on-market
O O
O O O
Oncology
Diabetes, dyslipidemia,
hypertension, HF, renal
disease, NASH
1bn people living with obesity Obesity is driver for >200 chronic diseases
O
AZD6234
LA Amylin
Phase I
O
BioPharmaceuticals
baxdrostat
ASI
Phase III
O O O
Opportunity beyond obesity to address cardiometabolic disease
Rare Disease
O
mitiperstat
MPO
Phase II
O O O OOO
AZD5462
ORXFP1
CEO Closing Remarks
Phase I
ECCOGENE
诚益生物
26 1. In China, Eccogene and AstraZeneca will operate under co-development and co-commercialisation agreement. GLP-1RA = glucagon-like peptide 1 receptor agonist; HF = heart failure; NASH = non-alcoholic steatohepatitis; LA =
long-acting; ASI = aldosterone synthase inhibitor; MPO = myeloperoxidase; ORXFP1 = oral relaxin family peptide receptor 1; oPCSK9 - oral proprotein convertase subtilisin/kexin type-9.
AZD0780
OPCSK9
Phase I
Strong track record in cardio renal metabolic diseases, opportunity for monotherapy and combinations
to address broad range of co-morbid diseasesView entire presentation