Kymera Investor Day Presentation Deck
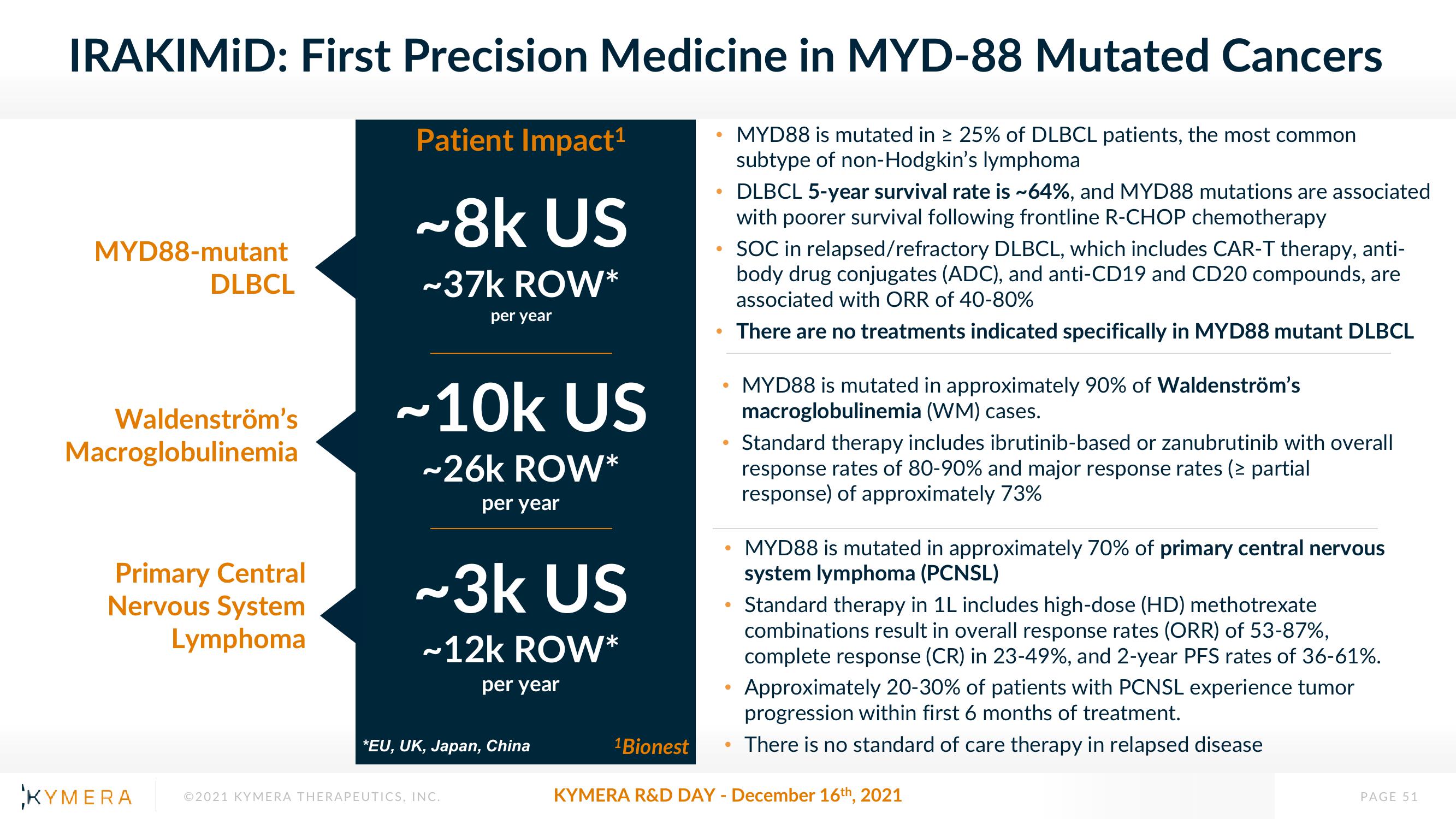
IRAKIMID: First Precision Medicine in MYD-88 Mutated Cancers
MYD88 is mutated in ≥ 25% of DLBCL patients, the most common
subtype of non-Hodgkin's lymphoma
MYD88-mutant
DLBCL
Waldenström's
Macroglobulinemia
Primary Central
Nervous System
Lymphoma
KYMERA
Patient Impact¹
~8k US
~37k ROW*
per year
~10k US
~26k ROW*
per year
~3k US
~12k ROW*
per year
*EU, UK, Japan, China
©2021 KYMERA THERAPEUTICS, INC.
●
• SOC in relapsed/refractory DLBCL, which includes CAR-T therapy, anti-
body drug conjugates (ADC), and anti-CD19 and CD20 compounds, are
associated with ORR of 40-80%
There are no treatments indicated specifically in MYD88 mutant DLBCL
●
●
●
DLBCL 5-year survival rate is ~64%, and MYD88 mutations are associated
with poorer survival following frontline R-CHOP chemotherapy
●
MYD88 is mutated in approximately 90% of Waldenström's
macroglobulinemia (WM) cases.
Standard therapy includes ibrutinib-based or zanubrutinib with overall
response rates of 80-90% and major response rates (≥ partial
response) of approximately 73%
MYD88 is mutated in approximately 70% of primary central nervous
system lymphoma (PCNSL)
Standard therapy in 1L includes high-dose (HD) methotrexate
combinations result in overall response rates (ORR) of 53-87%,
complete response (CR) in 23-49%, and 2-year PFS rates of 36-61%.
Approximately 20-30% of patients with PCNSL experience tumor
progression within first 6 months of treatment.
¹Bionest • There is no standard of care therapy in relapsed disease
KYMERA R&D DAY - December 16th, 2021
PAGE 51View entire presentation