BioNTech Investor Day Presentation Deck
BNT211
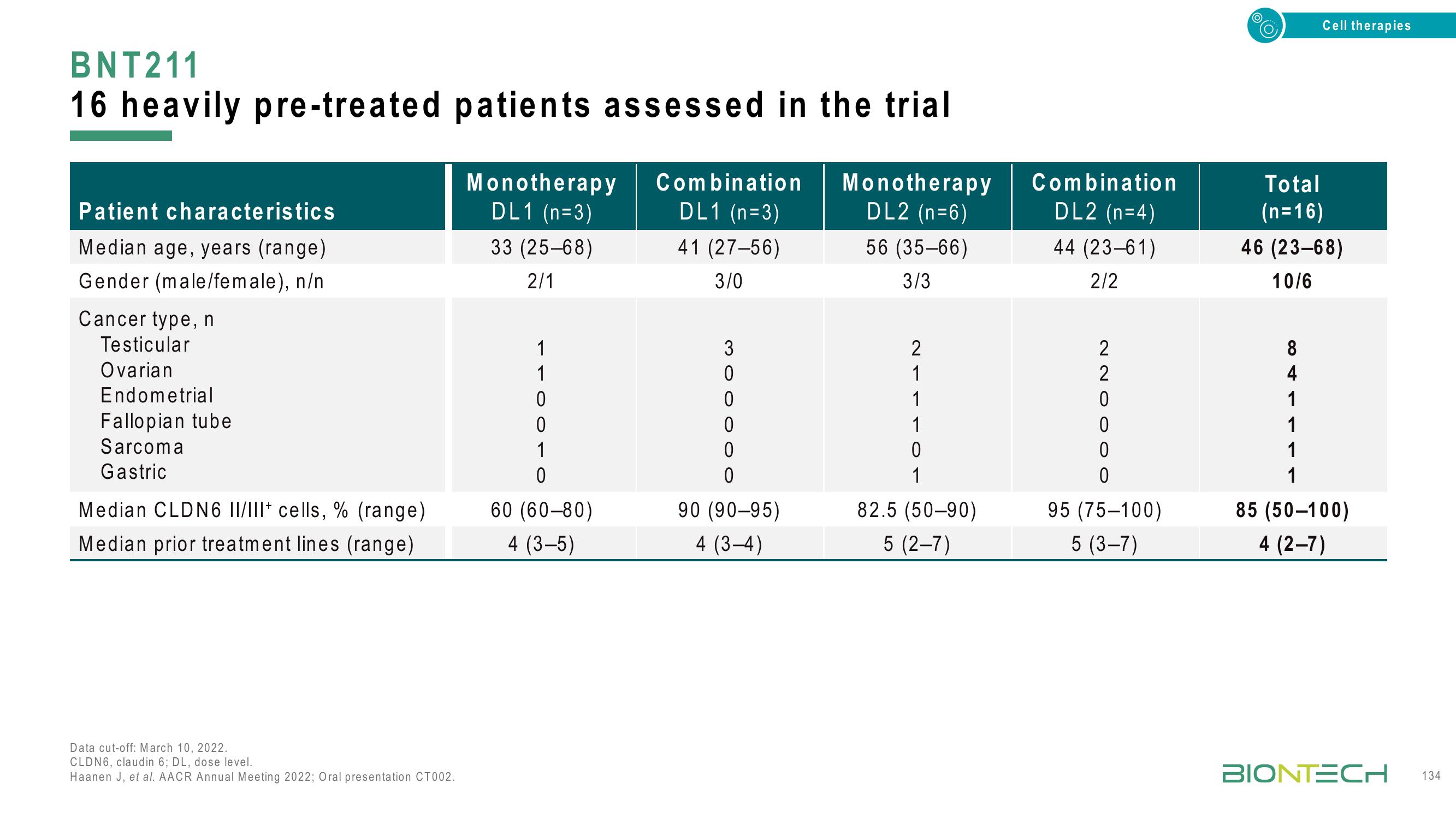
16 heavily pre-treated patients assessed in the trial
Patient characteristics
Median age, years (range)
Gender (male/female), n/n
Cancer type, n
Testicular
Ovarian
Endometrial
Fallopian tube
Sarcoma
Gastric
Median CLDN6 II/III+ cells, % (range)
Median prior treatment lines (range)
Data cut-off: March 10, 2022.
CLDN6, claudin 6; DL, dose level.
Haanen J, et al. AACR Annual Meeting 2022; Oral presentation CT002.
Monotherapy
DL1 (n=3)
33 (25-68)
2/1
1
1
0
0
1
0
60 (60-80)
4 (3-5)
Combination
DL1 (n=3)
41 (27-56)
3/0
3
O O O OW
0
0
0
0
0
90 (90-95)
4 (3-4)
Monotherapy
DL2 (n=6)
56 (35-66)
3/3
2
1
1
1
0
1
82.5 (50-90)
5 (2-7)
Combination
DL2 (n=4)
44 (23-61)
2/2
22000
0
95 (75-100)
5 (3-7)
Cell therapies
Total
(n=16)
46 (23-68)
10/6
8
4
1
1
1
1
85 (50-100)
4 (2-7)
BIONTECH
134View entire presentation