BioAtla IPO Presentation Deck
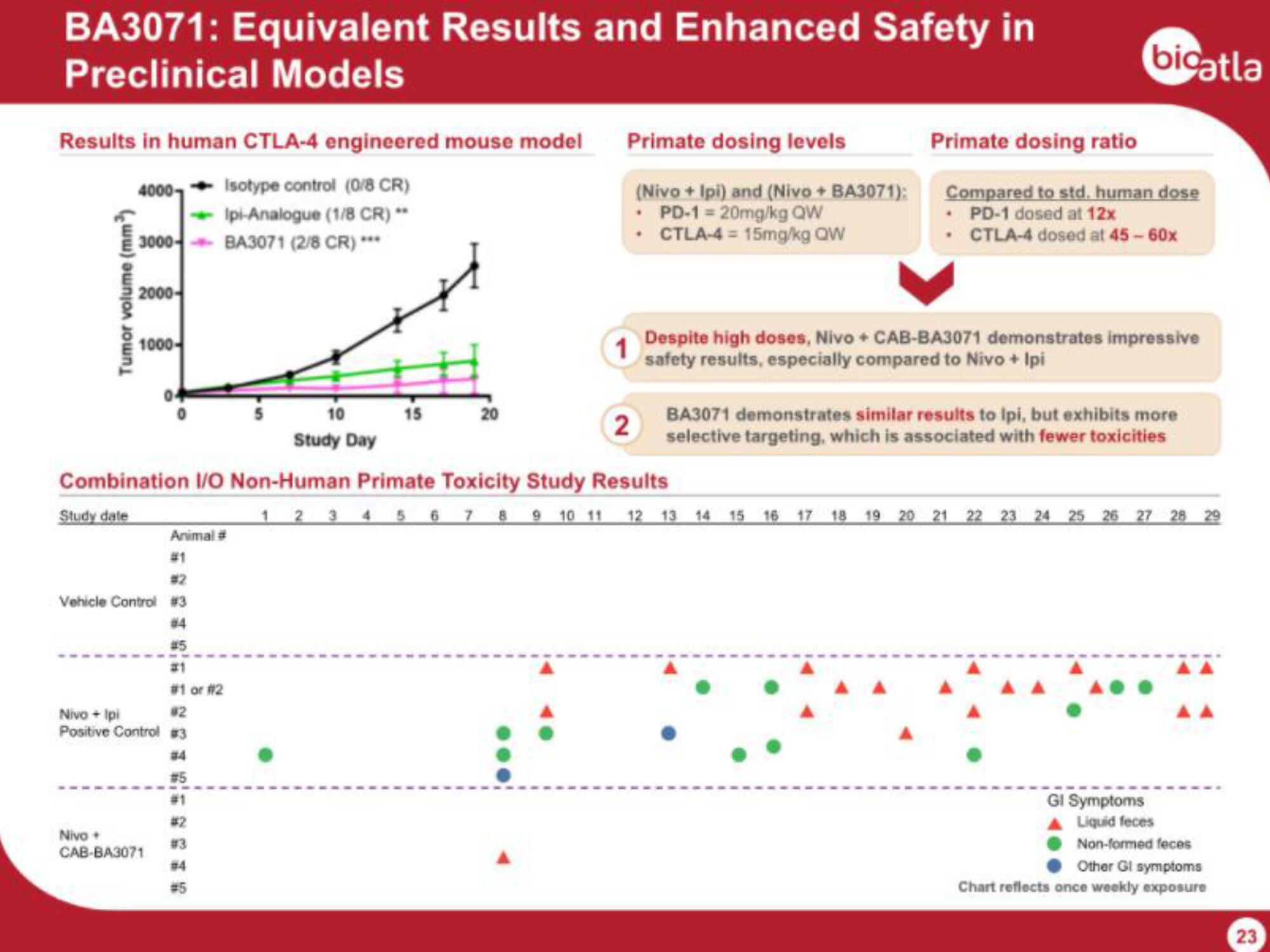
BA3071: Equivalent Results and Enhanced Safety in
Preclinical Models
Results in human CTLA-4 engineered mouse model
4000 Isotype control (0/8 CR)
Ipi-Analogue (1/8 CR)
3000- BA3071 (2/8 CR) ***
Tumor volume (mm)
2000-
1000-
Nivo +Ipi
Positive Control
Animal #
#1
Vehicle Control #3
Nivo
CAB-BA3071
#2
2
Combination I/O Non-Human Primate Toxicity Study Results
Study date
#4
#5
10
Study Day
#1 or #2
02
#3
#4
#5
#1
#2
# 3
#4
# 5
15
20
Primate dosing levels
(Nivo + Ipi) and (Nivo + BA3071):
• PD-1 = 20mg/kg QW
. CTLA-4= 15mg/kg QW
bicatla
Primate dosing ratio
Compared to std. human dose
• PD-1 dosed at 12x
. CTLA-4 dosed at 45 - 60x
1
Despite high doses, Nivo + CAB-BA3071 demonstrates impressive
safety results, especially compared to Nivo + Ipi
BA3071 demonstrates similar results to Ipi, but exhibits more
selective targeting, which is associated with fewer toxicities
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29
GI Symptoms
Liquid feces
Non-formed feces
Other Gl symptoms
Chart reflects once weekly exposure
23View entire presentation