WCG IPO Presentation Deck
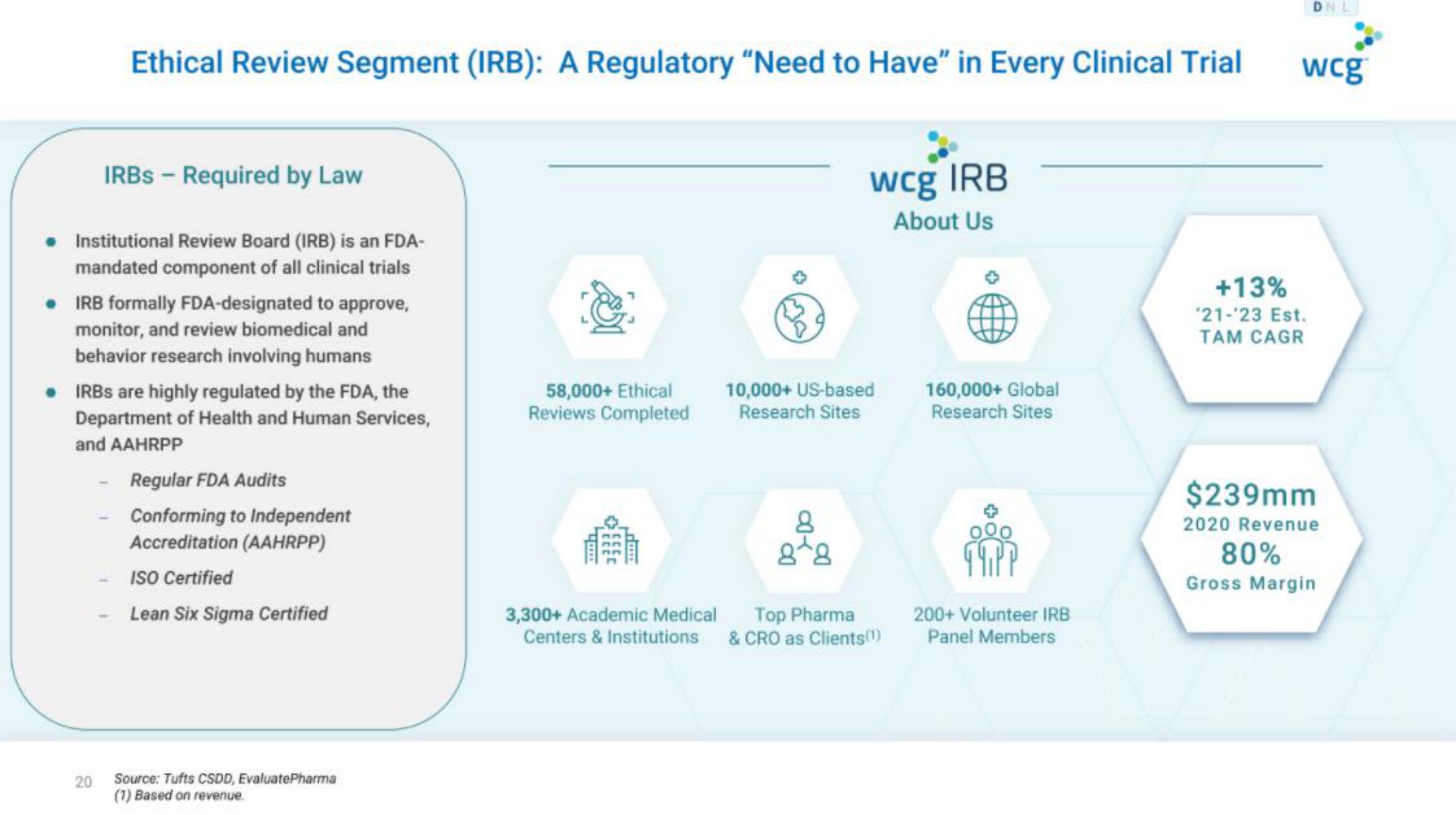
Ethical Review Segment (IRB): A Regulatory "Need to Have" in Every Clinical Trial
IRBS - Required by Law
● Institutional Review Board (IRB) is an FDA-
mandated component of all clinical trials
. IRB formally FDA-designated to approve,
monitor, and review biomedical and
behavior research involving humans
. IRBs are highly regulated by the FDA, the
Department of Health and Human Services,
and AAHRPP
20
Regular FDA Audits
Conforming to Independent
Accreditation (AAHRPP)
ISO Certified
Lean Six Sigma Certified
Source: Tufts CSDD, EvaluatePharma
(1) Based on revenue.
58,000+ Ethical
Reviews Completed
+
wcg IRB
About Us
10,000+ US-based
Research Sites
8+8
3,300+ Academic Medical Top Pharma
Centers & Institutions & CRO as Clients(¹)
160,000+ Global
Research Sites
200+ Volunteer IRB
Panel Members
DNL
wcg
+13%
*21-23 Est.
TAM CAGR
$239mm
2020 Revenue
80%
Gross MarginView entire presentation