BioAtla Investor Presentation Deck
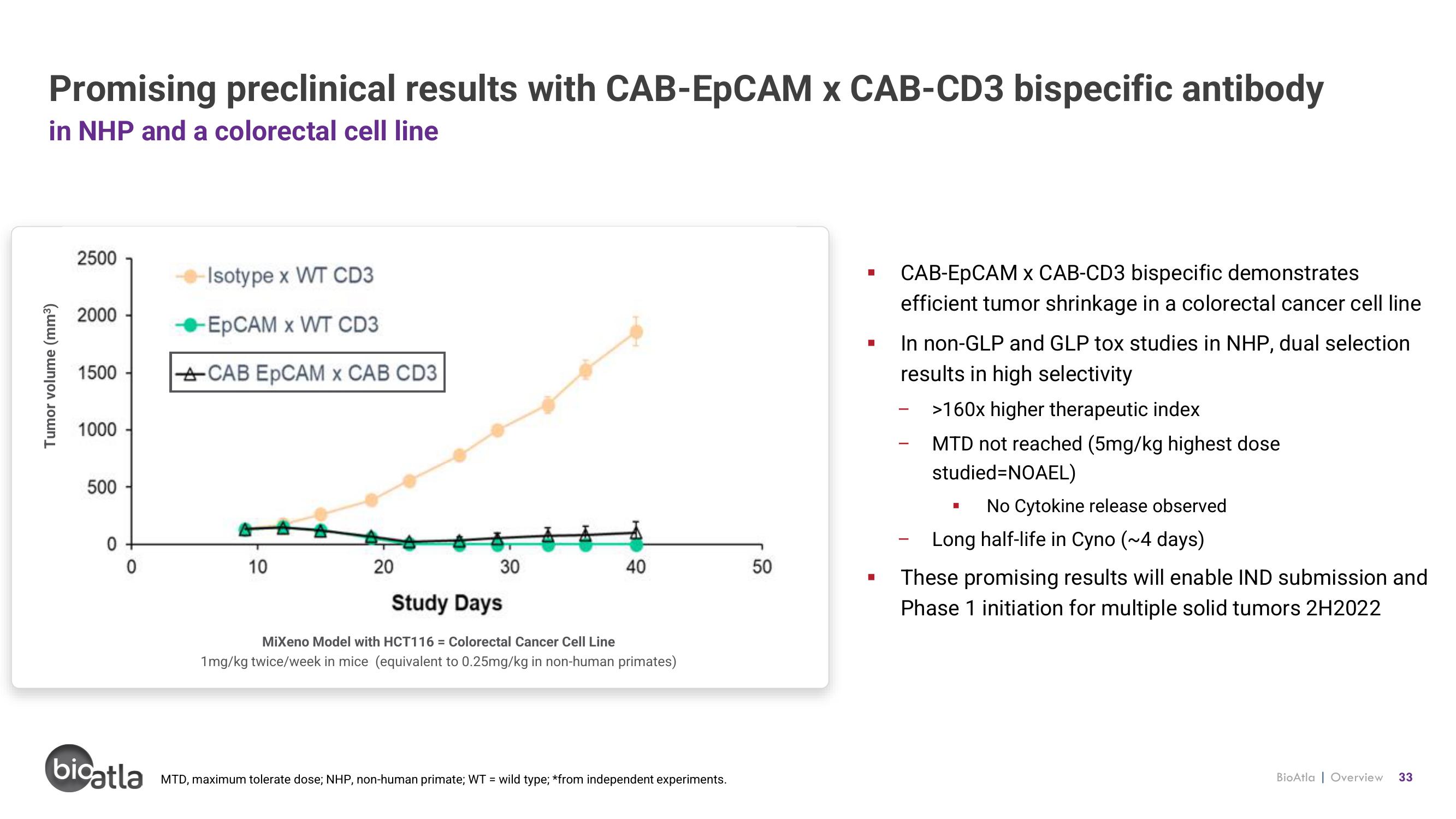
Promising preclinical results with CAB-EpCAM x CAB-CD3 bispecific antibody
in NHP and a colorectal cell line
Tumor volume (mm³)
2500
2000
1500
1000
500
0
T
0
bicatla
--Isotype x WT CD3
EpCAM x WT CD3
ACAB EpCAM x CAB CD3
10
20
30
40
Study Days
MiXeno Model with HCT116 = Colorectal Cancer Cell Line
1mg/kg twice/week in mice (equivalent to 0.25mg/kg in non-human primates)
MTD, maximum tolerate dose; NHP, non-human primate; WT = wild type; *from independent experiments.
50
■
CAB-EpCAM x CAB-CD3 bispecific demonstrates
efficient tumor shrinkage in a colorectal cancer cell line
In non-GLP and GLP tox studies in NHP, dual selection
results in high selectivity
>160x higher therapeutic index
MTD not reached (5mg/kg highest dose
studied=NOAEL)
No Cytokine release observed
Long half-life in Cyno (~4 days)
These promising results will enable IND submission and
Phase 1 initiation for multiple solid tumors 2H2022
-
■
BioAtla| Overview 33View entire presentation