BioAtla Investor Presentation Deck
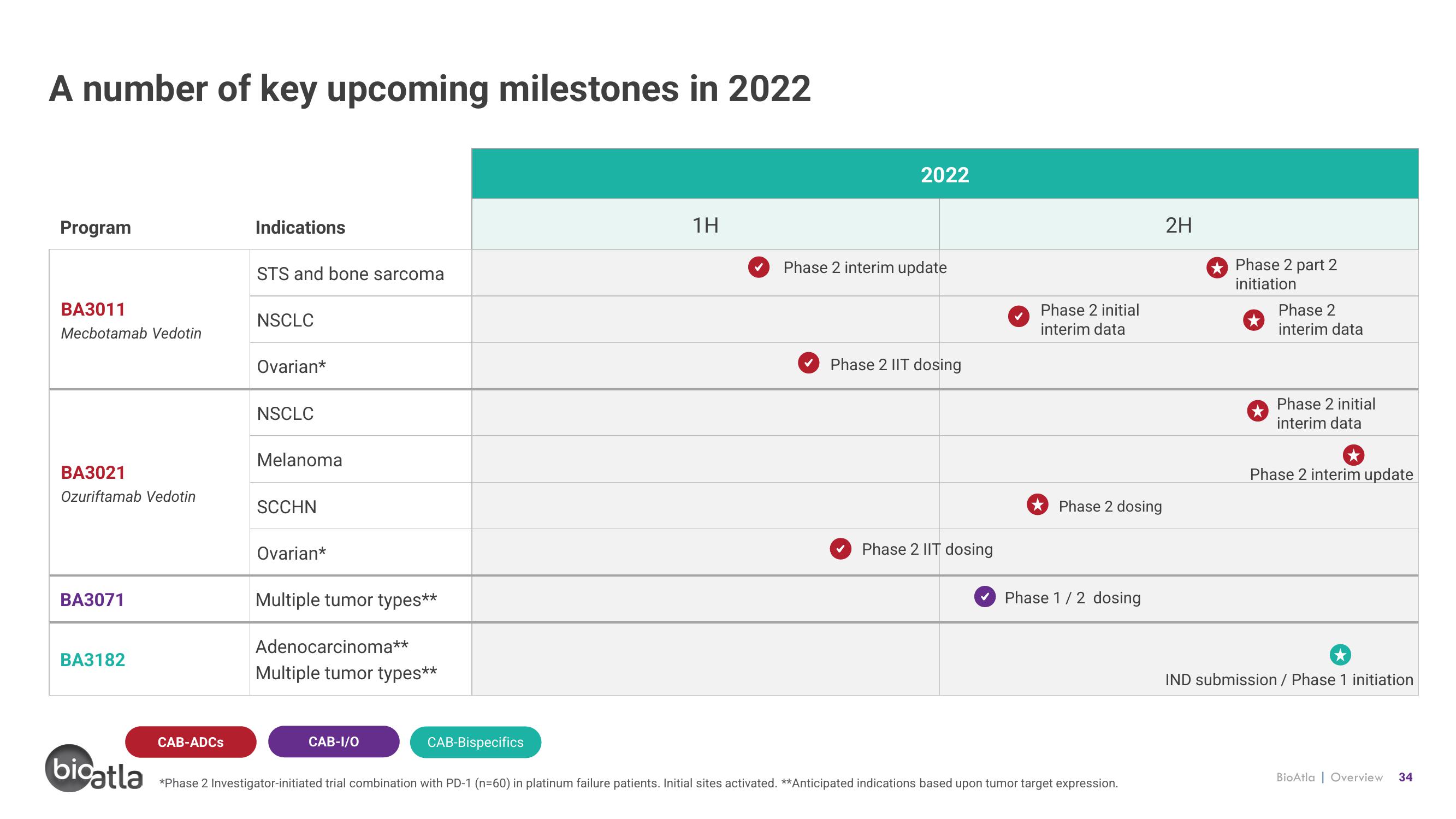
A number of key upcoming milestones in 2022
Program
BA3011
Mecbotamab Vedotin
BA3021
Ozuriftamab Vedotin
BA3071
BA3182
bicatla
CAB-ADCs
Indications
STS and bone sarcoma
NSCLC
Ovarian*
NSCLC
Melanoma
SCCHN
Ovarian*
Multiple tumor types**
Adenocarcinoma**
Multiple tumor types**
CAB-I/O
CAB-Bispecifics
1H
2022
Phase 2 interim update
Phase 2 IIT dosing
Phase 2 IIT dosing
Phase 2 initial
interim data
Phase 2 dosing
Phase 1/2 dosing
*Phase 2 Investigator-initiated trial combination with PD-1 (n=60) in platinum failure patients. Initial sites activated. **Anticipated indications based upon tumor target expression.
2H
Phase 2 part 2
initiation
Phase 2
interim data
Phase 2 initial
interim data
Phase 2 interim update
IND submission / Phase 1 initiation
BioAtla| Overview 34View entire presentation