Imara M&A
Tumor Volume
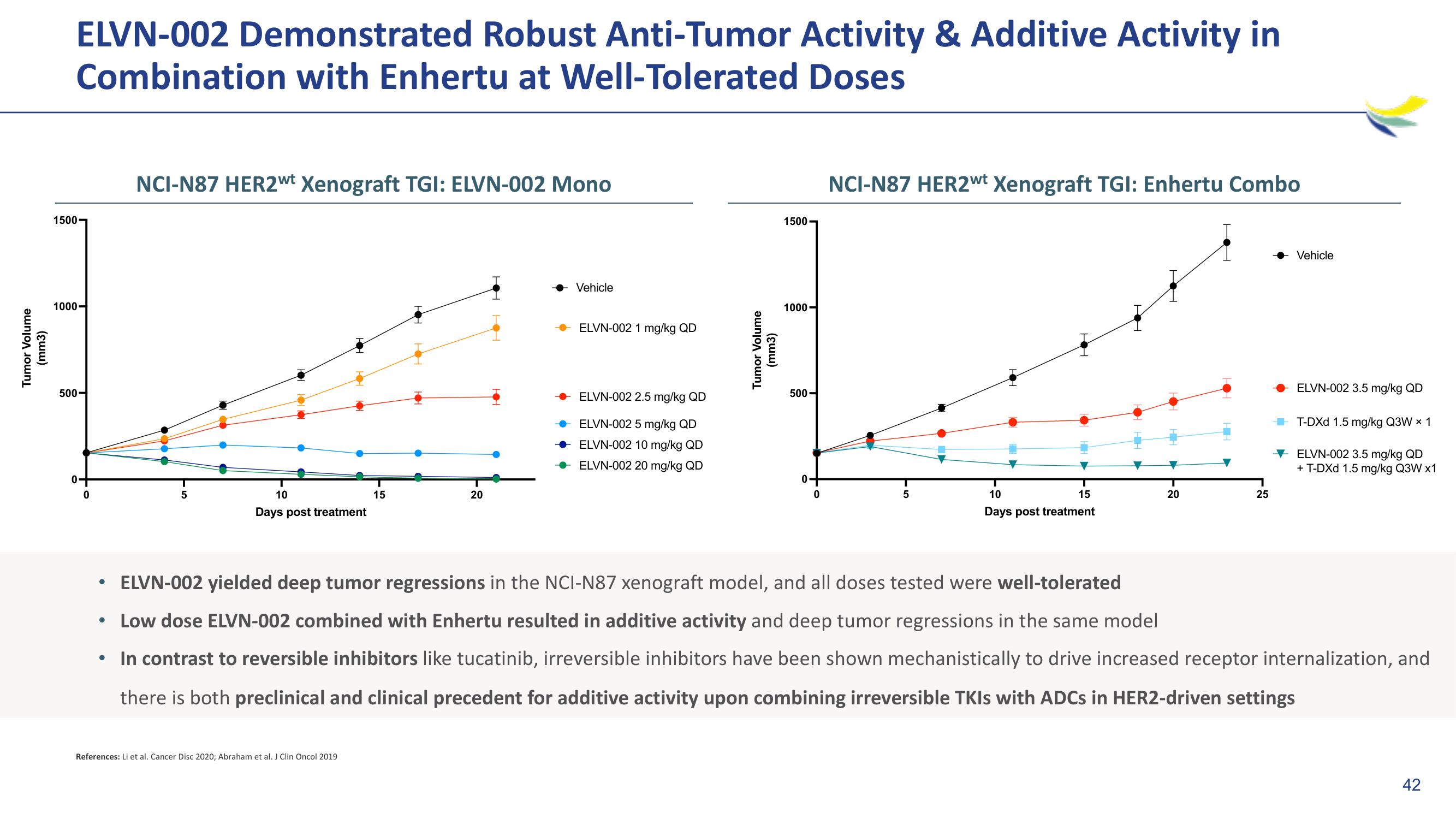
ELVN-002 Demonstrated Robust Anti-Tumor Activity & Additive Activity in
Combination with Enhertu at Well-Tolerated Doses
(mm3)
1500-
LE
10
Days post treatment
1000-
500-
0-
0
NCI-N87 HER2wt Xenograft TGI: ELVN-002 Mono
●
●
5
15
References: Li et al. Cancer Disc 2020; Abraham et al. J Clin Oncol 2019
20
Vehicle
ELVN-002 1 mg/kg QD
ELVN-002 2.5 mg/kg QD
ELVN-002 5 mg/kg QD
ELVN-002 10 mg/kg QD
ELVN-002 20 mg/kg QD
Tumor Volume
(mm3)
1500-
1000-
500-
0
NCI-N87 HER2wt Xenograft TGI: Enhertu Combo
15
10
15
Days post treatment
+
20
25
Vehicle
ELVN-002 yielded deep tumor regressions in the NCI-N87 xenograft model, and all doses tested were well-tolerated
• Low dose ELVN-002 combined with Enhertu resulted in additive activity and deep tumor regressions in the same model
In contrast to reversible inhibitors like tucatinib, irreversible inhibitors have been shown mechanistically to drive increased receptor internalization, and
there is both preclinical and clinical precedent for additive activity upon combining irreversible TKIs with ADCs in HER2-driven settings
ELVN-002 3.5 mg/kg QD
T-DXd 1.5 mg/kg Q3W x 1
ELVN-002 3.5 mg/kg QD
+ T-DXd 1.5 mg/kg Q3W x1
42View entire presentation