BioNTech Investor Day Presentation Deck
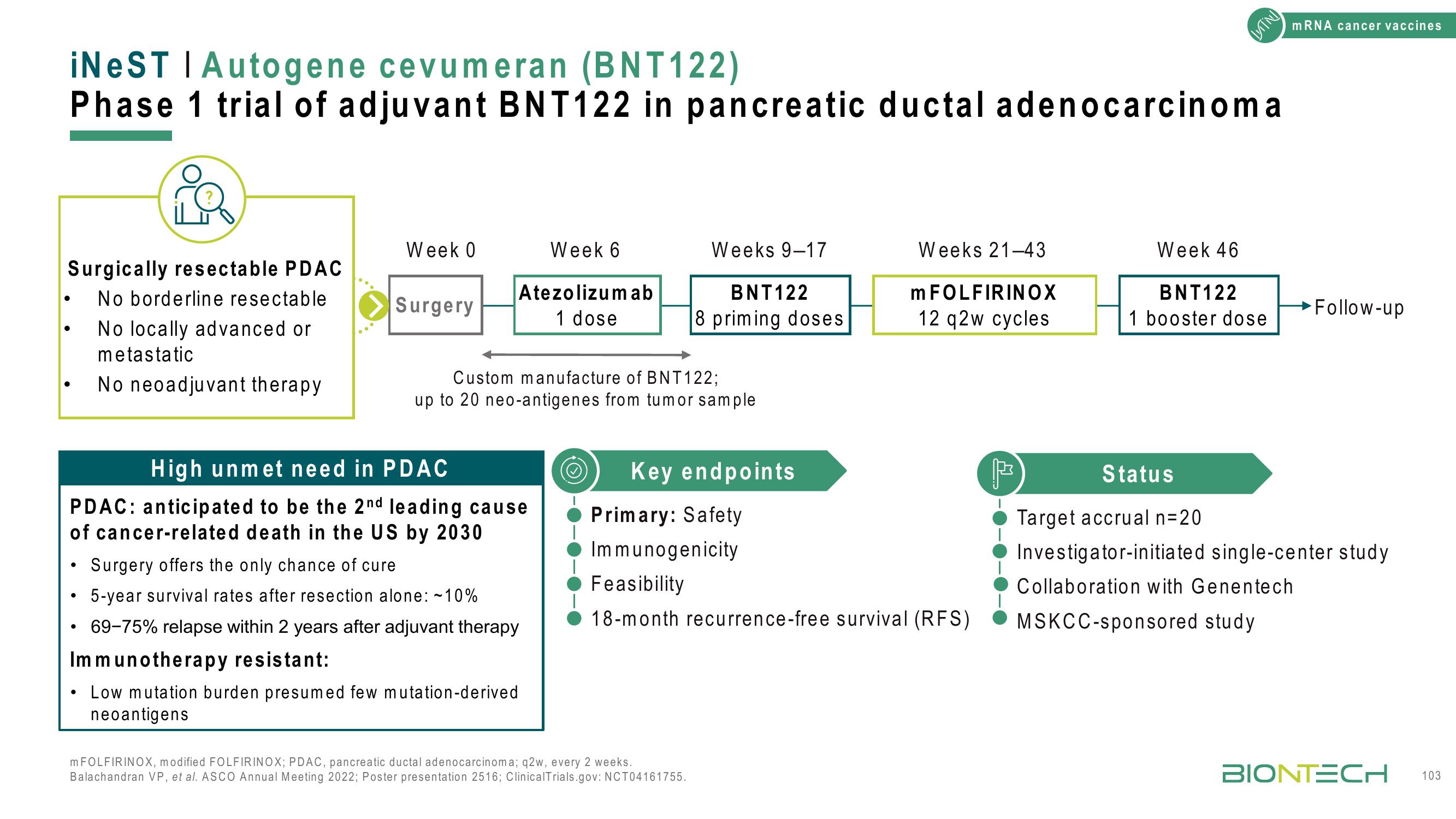
iNeST | Autogene cevumeran (BNT122)
Phase 1 trial of adjuvant BNT122 in pancreatic ductal adenocarcinoma
Surgically resectable PDAC
No borderline resectable
No locally advanced or
metastatic
No neoadjuvant therapy
●
Week 0
●
Surgery
High unmet need in PDAC
PDAC: anticipated to be the 2nd leading cause
of cancer-related death in the US by 2030
●
Week 6
Atezolizumab
1 dose
Surgery offers the only chance of cure
5-year survival rates after resection alone: ~10%
• 69-75% relapse within 2 years after adjuvant therapy
Immunotherapy resistant:
Low mutation burden presumed few mutation-derived
neoantigens
Custom manufacture of BNT122;
up to 20 neo-antigenes from tumor sample
Weeks 9-17
BNT122
8 priming doses
Key endpoints
mFOLFIRINOX, modified FOLFIRINOX; PDAC, pancreatic ductal adenocarcinoma; q2w, every 2 weeks.
Balachandran VP, et al. ASCO Annual Meeting 2022; Poster presentation 2516; Clinical Trials.gov: NCT04161755.
Weeks 21-43
m FOLFIRINOX
12 q2w cycles
Primary: Safety
Immunogenicity
Feasibility
18-month recurrence-free survival (RFS)
Week 46
NUM
BNT122
1 booster dose
mRNA cancer vaccines
→Follow-up
Status
Target accrual n=20
Investigator-initiated single-center study
Collaboration with Genentech
MSKCC-sponsored study
BIONTECH
103View entire presentation