Ocuphire Pharma Investor Day Presentation Deck
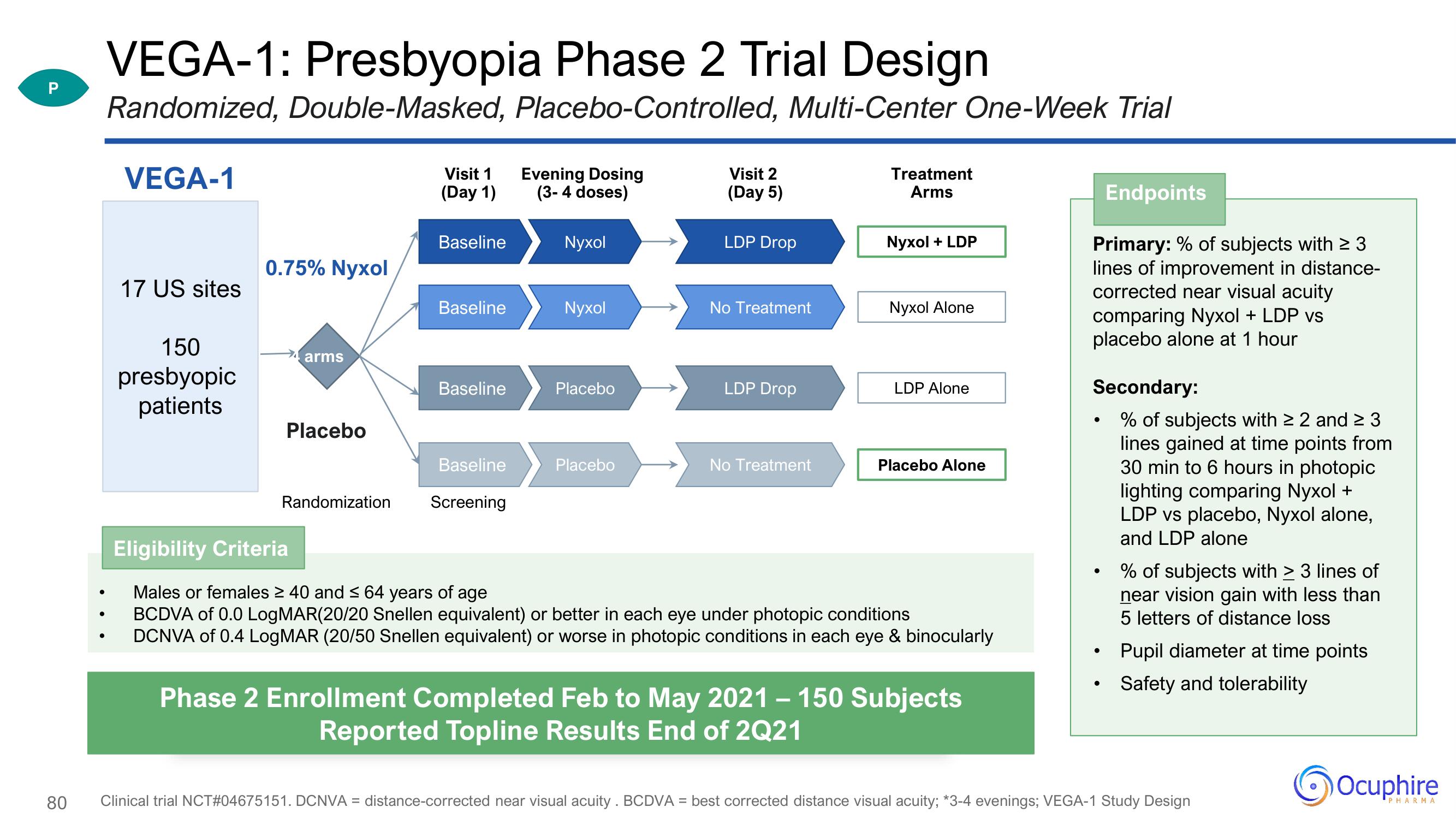
VEGA-1: Presbyopia Phase 2 Trial Design
P Randomized, Double-Masked, Placebo-Controlled, Multi-Center One-Week Trial
80
●
●
●
VEGA-1
17 US sites
150
presbyopic
patients
0.75% Nyxol
arms
Placebo
Randomization
Visit 1
(Day 1)
Baseline
Baseline
Baseline
Baseline
Screening
Evening Dosing
(3-4 doses)
Nyxol
Nyxol
Placebo
Placebo
Visit 2
(Day 5)
LDP Drop
No Treatment
LDP Drop
No Treatment
Treatment
Arms
Nyxol + LDP
Nyxol Alone
LDP Alone
Placebo Alone
Eligibility Criteria
Males or females ≥ 40 and ≤ 64 years of age
BCDVA of 0.0 LogMAR(20/20 Snellen equivalent) or better in each eye under photopic conditions
DCNVA of 0.4 LogMAR (20/50 Snellen equivalent) or worse in photopic conditions in each eye & binocularly
Phase 2 Enrollment Completed Feb to May 2021 – 150 Subjects
Reported Topline Results End of 2Q21
Endpoints
Primary: % of subjects with ≥ 3
lines of improvement in distance-
corrected near visual acuity
comparing Nyxol + LDP vs
placebo alone at 1 hour
Secondary:
• % of subjects with ≥ 2 and ≥ 3
lines gained at time points from
30 min to 6 hours in photopic
lighting comparing Nyxol +
LDP vs placebo, Nyxol alone,
and LDP alone
●
% of subjects with > 3 lines of
near vision gain with less than
5 letters of distance loss
Pupil diameter at time points
Safety and tolerability
Clinical trial NCT #04675151. DCNVA = distance-corrected near visual acuity. BCDVA = best corrected distance visual acuity; *3-4 evenings; VEGA-1 Study Design
Ocuphire
PHARMAView entire presentation