ATAI Investor Presentation Deck
RL-007: a de-risked pro-cognitive neuromodulator with excellent tolerability
in humans
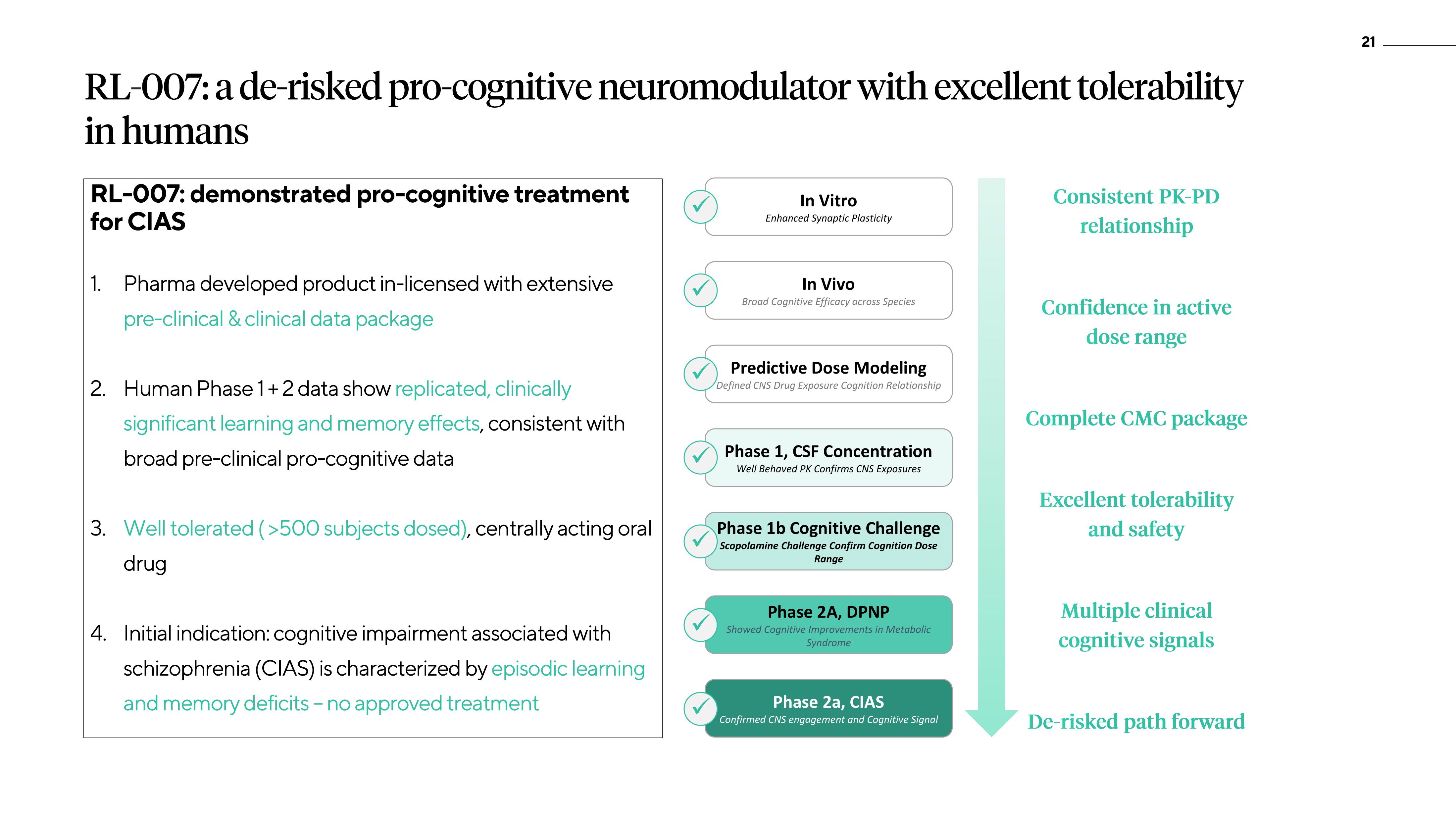
RL-007: demonstrated pro-cognitive treatment
for CIAS
1. Pharma developed product in-licensed with extensive
pre-clinical & clinical data package
2. Human Phase 1+2 data show replicated, clinically
significant learning and memory effects, consistent with
broad pre-clinical pro-cognitive data
3. Well tolerated (>500 subjects dosed), centrally acting oral
drug
4. Initial indication: cognitive impairment associated with
schizophrenia (CIAS) is characterized by episodic learning
and memory deficits - no approved treatment
In Vitro
Enhanced Synaptic Plasticity
In Vivo
Broad Cognitive Efficacy across Species
Predictive Dose Modeling
Defined CNS Drug Exposure Cognition Relationship
Phase 1, CSF Concentration
Well Behaved PK Confirms CNS Exposures
Phase 1b Cognitive Challenge
Scopolamine Challenge Confirm Cognition Dose
Range
Phase 2A, DPNP
Showed Cognitive Improvements in Metabolic
Syndrome
Phase 2a, CIAS
Confirmed CNS engagement and Cognitive Signal
Consistent PK-PD
relationship
Confidence in active
dose range
Complete CMC package
Excellent tolerability
and safety
Multiple clinical
cognitive signals
De-risked path forward
21View entire presentation