Corporate Presentation
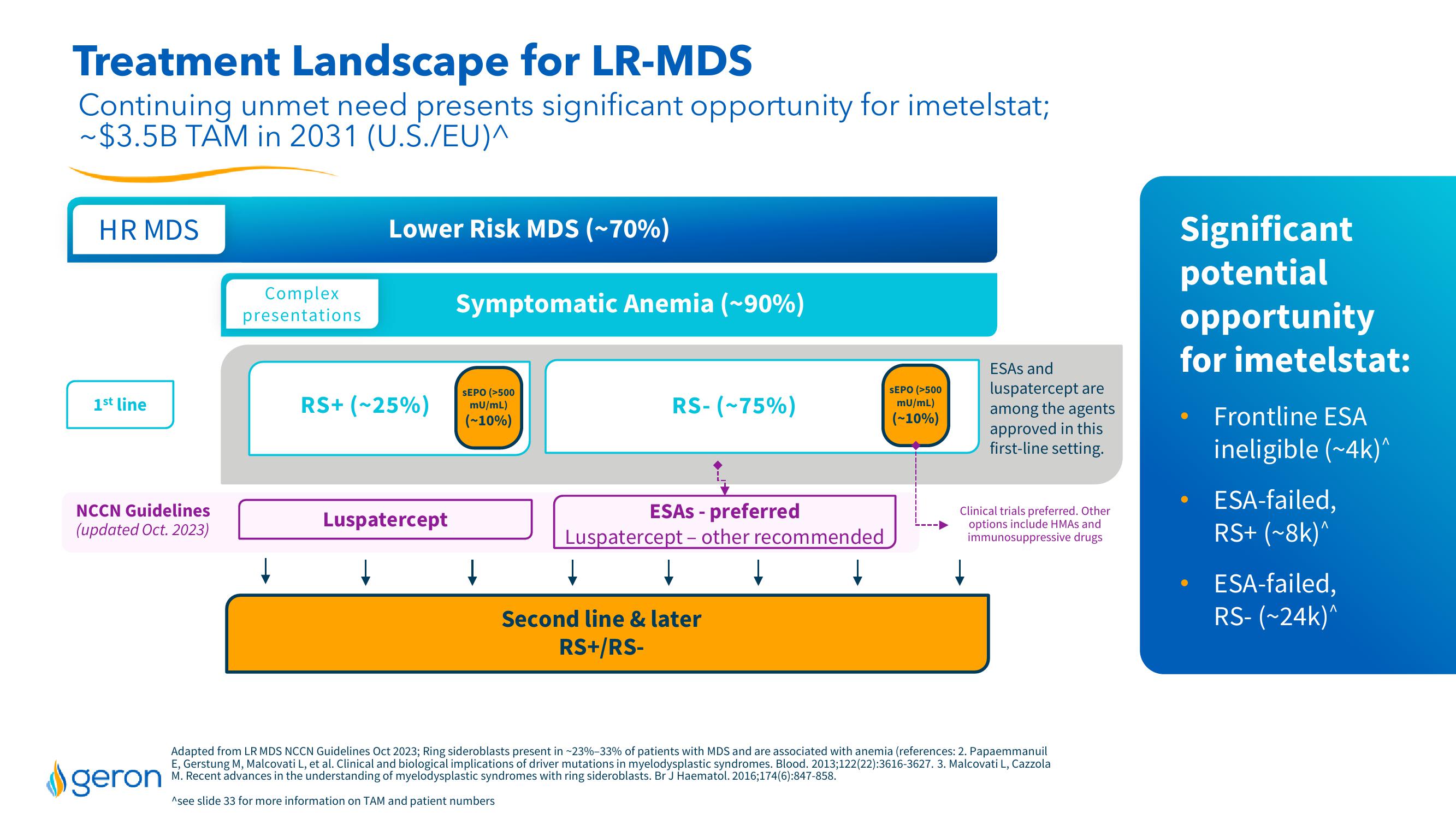
Treatment Landscape for LR-MDS
Continuing unmet need presents significant opportunity for imetelstat;
~$3.5B TAM in 2031 (U.S./EU)^
HR MDS
1st line
NCCN Guidelines
(updated Oct. 2023)
geron
Complex
presentations
↓
Lower Risk MDS (~70%)
RS+ (~25%)
Luspatercept
↓
Symptomatic Anemia (~90%)
SEPO (>500
mU/mL)
(-10%)
↓
RS-(-75%)
ESAS - preferred
Luspatercept - other recommended
↓
↓
Second line & later
RS+/RS-
SEPO (>500
mU/mL)
(-10%)
ESAS and
luspatercept are
among the agents
approved in this
first-line setting.
Clinical trials preferred. Other
options include HMAS and
immunosuppressive drugs
Adapted from LR MDS NCCN Guidelines Oct 2023; Ring sideroblasts present in ~23%-33% of patients with MDS and are associated with anemia (references: 2. Papaemmanuil
E, Gerstung M, Malcovati L, et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood. 2013;122 (22):3616-3627. 3. Malcovati L, Cazzola
M. Recent advances in the understanding of myelodysplastic syndromes with ring sideroblasts. Br J Haematol. 2016;174(6):847-858.
^see slide 33 for more information on TAM and patient numbers
Significant
potential
opportunity
for imetelstat:
Frontline ESA
ineligible (~4k)^
ESA-failed,
RS+ (~8k)^
ESA-failed,
RS-(~24k)^View entire presentation